Enterprise Image-Viewing System Receives FDA Clearance for Mobile Diagnosis on All Modalities
|
By MedImaging International staff writers Posted on 23 Apr 2014 |
An enterprise image-viewing system is now cleared in the United States for diagnosis on mobile devices, for all imaging modalities (except mammography).
Calgary Scientific, Inc. (Calgary, AB, USA) recently reported their latest Class II clearance from the US Food and Drug Administration (FDA). Calgary Scientific worked closely with a number of Radiologists at a world renowned healthcare organization to clinically validate the solution prior to submitting to the FDA. This builds upon their previous clearances for diagnosis using web, iOS, and Android devices.
“We chose to implement ResolutionMD because it gives us the ability to quickly disseminate the images from our Pediatric Imaging Departments so that clinicians can provide the fastest and best care and treatment to our patients,” said Stuart Royal, MS, MD, radiologist-in-chief at Children’s Hospital of Alabama. “Their fully accredited, diagnostic image quality and secure solution gives us the flexibility to make clinical decisions from any device type, portable or desktop throughout our healthcare system and off-site.”
The FDA’s safety and effectiveness review of ResolutionMD found it to be substantially equivalent to PACS or dedicated diagnostic workstations, giving practitioners access to advanced image storage systems and diagnostic tool sets on-the-go. Gaining this clearance means a healthcare enterprise, across all departments, can feel confident in using ResolutionMD to perform a clinical diagnosis on web, iOS and Android devices, as it has been through a rigorous testing process, as agreed to by the FDA.
“We are excited to deliver this clearance to the institutions that rely on ResolutionMD to improve patient care,” said Pierre Lemire, president and CTO of Calgary Scientific. “This is also an important advancement for all our valued partners including those who deliver custom-branded solutions, as they will be added under the Calgary Scientific registration with the FDA.”
ResolutionMD enables doctors to securely view patient images and reports from a wide variety of computers and mobile devices, collaborate with other practitioners, and diagnose from any location. The FDA-cleared, Health Canada-licensed, and CE marked mobile medical diagnosis software can be integrated into any EMR and easily plugs into existing distributed storage systems.
ResolutionMD’s federated approach is an important differentiator from other solutions as highly sensitive data is never moved to a device and no additional data storage locations are created. ResolutionMD is published in twelve languages and is currently installed in leading healthcare institutions around the world via a network of more than thirty-five world class healthcare partners.
Calgary Scientific is a developer of web and mobile diagnostic medical imaging and cloud-enabling solutions.
Related Links:
Calgary Scientific
Calgary Scientific, Inc. (Calgary, AB, USA) recently reported their latest Class II clearance from the US Food and Drug Administration (FDA). Calgary Scientific worked closely with a number of Radiologists at a world renowned healthcare organization to clinically validate the solution prior to submitting to the FDA. This builds upon their previous clearances for diagnosis using web, iOS, and Android devices.
“We chose to implement ResolutionMD because it gives us the ability to quickly disseminate the images from our Pediatric Imaging Departments so that clinicians can provide the fastest and best care and treatment to our patients,” said Stuart Royal, MS, MD, radiologist-in-chief at Children’s Hospital of Alabama. “Their fully accredited, diagnostic image quality and secure solution gives us the flexibility to make clinical decisions from any device type, portable or desktop throughout our healthcare system and off-site.”
The FDA’s safety and effectiveness review of ResolutionMD found it to be substantially equivalent to PACS or dedicated diagnostic workstations, giving practitioners access to advanced image storage systems and diagnostic tool sets on-the-go. Gaining this clearance means a healthcare enterprise, across all departments, can feel confident in using ResolutionMD to perform a clinical diagnosis on web, iOS and Android devices, as it has been through a rigorous testing process, as agreed to by the FDA.
“We are excited to deliver this clearance to the institutions that rely on ResolutionMD to improve patient care,” said Pierre Lemire, president and CTO of Calgary Scientific. “This is also an important advancement for all our valued partners including those who deliver custom-branded solutions, as they will be added under the Calgary Scientific registration with the FDA.”
ResolutionMD enables doctors to securely view patient images and reports from a wide variety of computers and mobile devices, collaborate with other practitioners, and diagnose from any location. The FDA-cleared, Health Canada-licensed, and CE marked mobile medical diagnosis software can be integrated into any EMR and easily plugs into existing distributed storage systems.
ResolutionMD’s federated approach is an important differentiator from other solutions as highly sensitive data is never moved to a device and no additional data storage locations are created. ResolutionMD is published in twelve languages and is currently installed in leading healthcare institutions around the world via a network of more than thirty-five world class healthcare partners.
Calgary Scientific is a developer of web and mobile diagnostic medical imaging and cloud-enabling solutions.
Related Links:
Calgary Scientific
Latest Imaging IT News
- New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
- Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
- AI-Based Mammography Triage Software Helps Dramatically Improve Interpretation Process
- Artificial Intelligence (AI) Program Accurately Predicts Lung Cancer Risk from CT Images
- Image Management Platform Streamlines Treatment Plans
- AI-Based Technology for Ultrasound Image Analysis Receives FDA Approval
- AI Technology for Detecting Breast Cancer Receives CE Mark Approval
- Digital Pathology Software Improves Workflow Efficiency
- Patient-Centric Portal Facilitates Direct Imaging Access
- New Workstation Supports Customer-Driven Imaging Workflow
Channels
Radiography
view channel
Machine Learning Algorithm Identifies Cardiovascular Risk from Routine Bone Density Scans
A new study published in the Journal of Bone and Mineral Research reveals that an automated machine learning program can predict the risk of cardiovascular events and falls or fractures by analyzing bone... Read more
AI Improves Early Detection of Interval Breast Cancers
Interval breast cancers, which occur between routine screenings, are easier to treat when detected earlier. Early detection can reduce the need for aggressive treatments and improve the chances of better outcomes.... Read more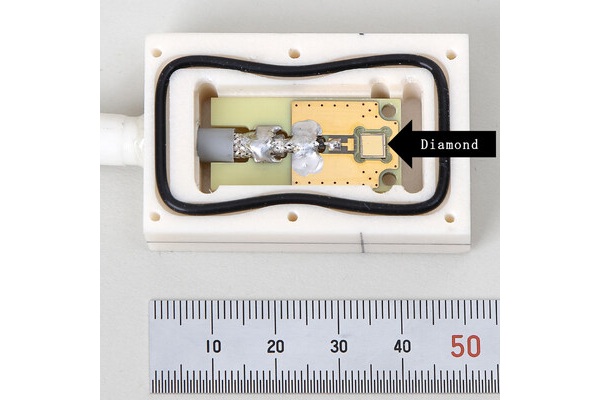
World's Largest Class Single Crystal Diamond Radiation Detector Opens New Possibilities for Diagnostic Imaging
Diamonds possess ideal physical properties for radiation detection, such as exceptional thermal and chemical stability along with a quick response time. Made of carbon with an atomic number of six, diamonds... Read moreMRI
view channel
New MRI Technique Reveals Hidden Heart Issues
Traditional exercise stress tests conducted within an MRI machine require patients to lie flat, a position that artificially improves heart function by increasing stroke volume due to gravity-driven blood... Read more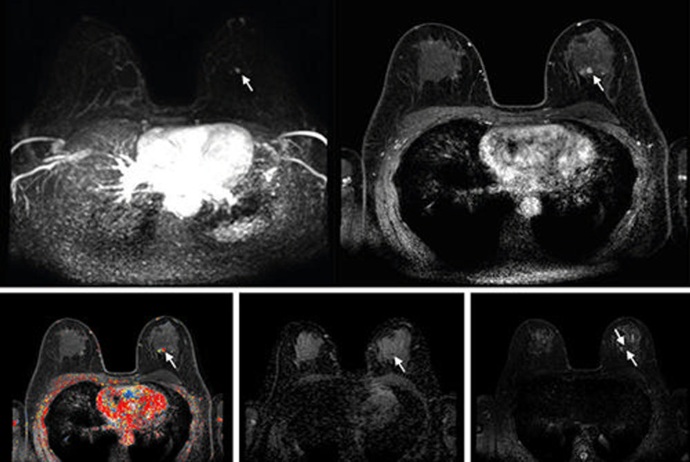
Shorter MRI Exam Effectively Detects Cancer in Dense Breasts
Women with extremely dense breasts face a higher risk of missed breast cancer diagnoses, as dense glandular and fibrous tissue can obscure tumors on mammograms. While breast MRI is recommended for supplemental... Read moreUltrasound
view channel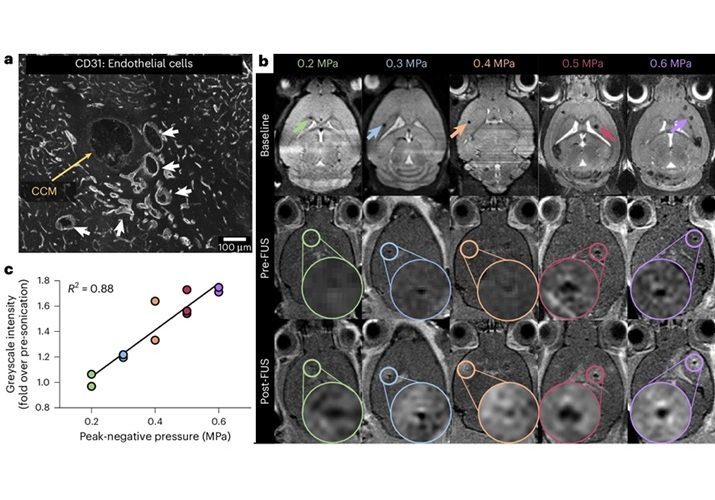
New Incision-Free Technique Halts Growth of Debilitating Brain Lesions
Cerebral cavernous malformations (CCMs), also known as cavernomas, are abnormal clusters of blood vessels that can grow in the brain, spinal cord, or other parts of the body. While most cases remain asymptomatic,... Read more.jpeg)
AI-Powered Lung Ultrasound Outperforms Human Experts in Tuberculosis Diagnosis
Despite global declines in tuberculosis (TB) rates in previous years, the incidence of TB rose by 4.6% from 2020 to 2023. Early screening and rapid diagnosis are essential elements of the World Health... Read moreNuclear Medicine
view channel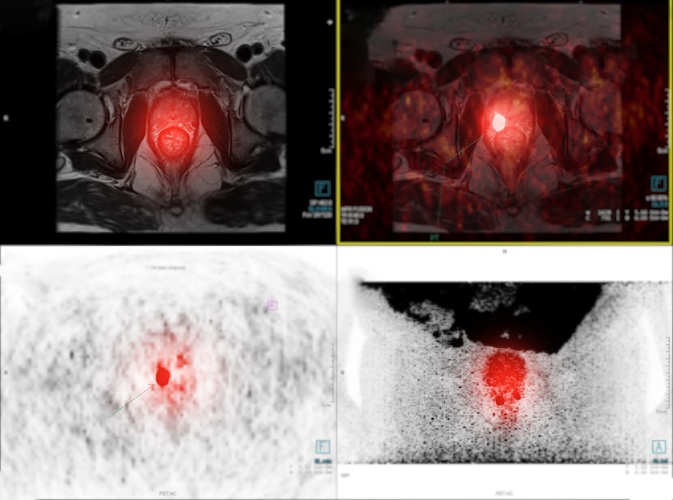
New Imaging Approach Could Reduce Need for Biopsies to Monitor Prostate Cancer
Prostate cancer is the second leading cause of cancer-related death among men in the United States. However, the majority of older men diagnosed with prostate cancer have slow-growing, low-risk forms of... Read more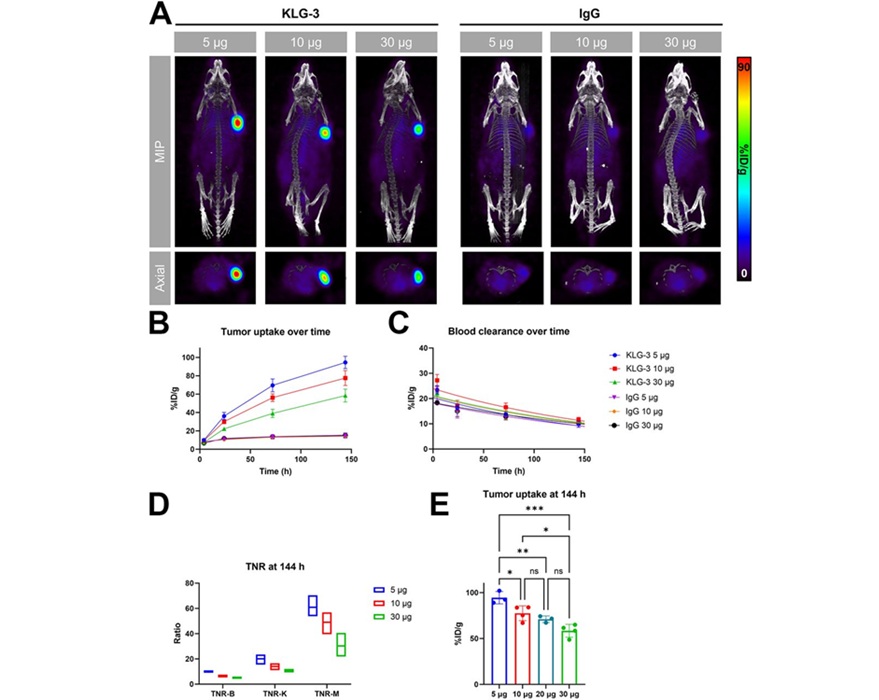
Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
Interleukin-13 receptor α-2 (IL13Rα2) is a cell surface receptor commonly found in solid tumors such as glioblastoma, melanoma, and breast cancer. It is minimally expressed in normal tissues, making it... Read moreGeneral/Advanced Imaging
view channel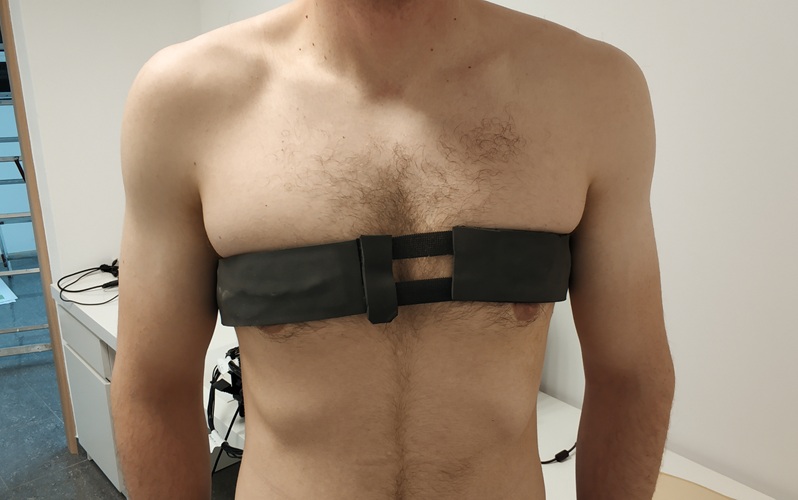
First-Of-Its-Kind Wearable Device Offers Revolutionary Alternative to CT Scans
Currently, patients with conditions such as heart failure, pneumonia, or respiratory distress often require multiple imaging procedures that are intermittent, disruptive, and involve high levels of radiation.... Read more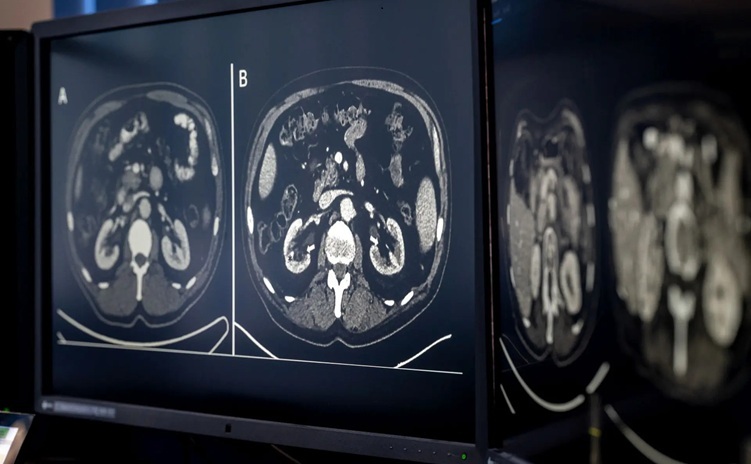
AI-Based CT Scan Analysis Predicts Early-Stage Kidney Damage Due to Cancer Treatments
Radioligand therapy, a form of targeted nuclear medicine, has recently gained attention for its potential in treating specific types of tumors. However, one of the potential side effects of this therapy... Read moreIndustry News
view channel
GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
GE HealthCare (Chicago, IL, USA) has entered into a collaboration with NVIDIA (Santa Clara, CA, USA), expanding the existing relationship between the two companies to focus on pioneering innovation in... Read more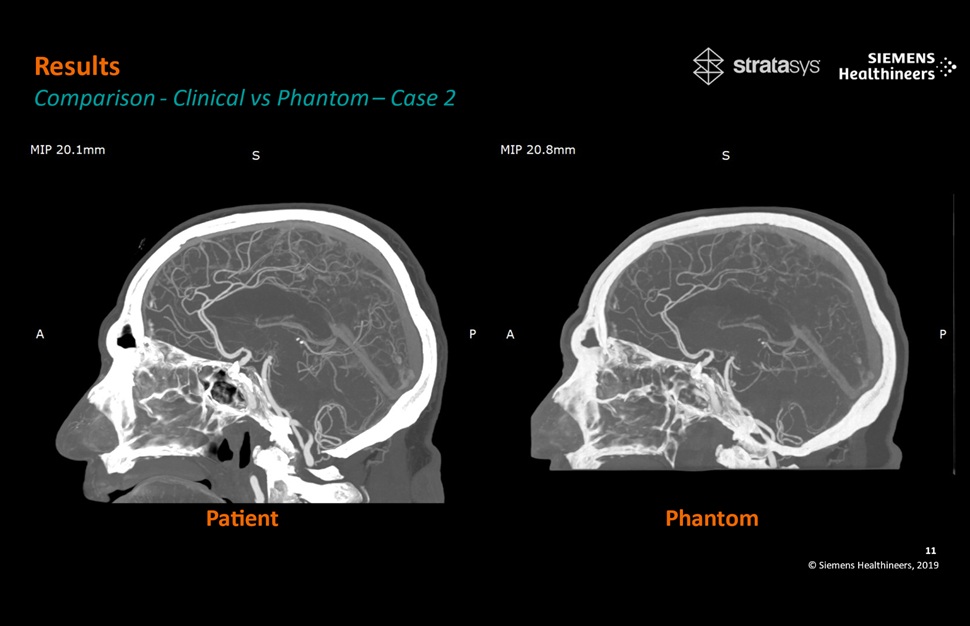
Patient-Specific 3D-Printed Phantoms Transform CT Imaging
New research has highlighted how anatomically precise, patient-specific 3D-printed phantoms are proving to be scalable, cost-effective, and efficient tools in the development of new CT scan algorithms... Read more
Siemens and Sectra Collaborate on Enhancing Radiology Workflows
Siemens Healthineers (Forchheim, Germany) and Sectra (Linköping, Sweden) have entered into a collaboration aimed at enhancing radiologists' diagnostic capabilities and, in turn, improving patient care... Read more