Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
Posted on 23 Apr 2025
Interleukin-13 receptor α-2 (IL13Rα2) is a cell surface receptor commonly found in solid tumors such as glioblastoma, melanoma, and breast cancer. It is minimally expressed in normal tissues, making it an ideal target for non-invasive and specific detection of tumors. However, despite its potential, there are currently no IL13Rα2-targeting antibodies available for diagnostic or therapeutic (theranostic) use in clinical settings. A newly developed radiolabeled antibody targeting the IL13Rα2 cancer antigen has shown high specificity, binding exclusively to cancer cells while avoiding interaction with the related IL13Rα1 antigen, which is more broadly expressed in healthy tissues. When tested across various cancer types, the radiolabeled antibody was effective at identifying tumors at a low injected mass dose, suggesting its potential for use in radioimmunotherapy applications.
In a study published in The Journal of Nuclear Medicine, researchers from the University of Texas MD Anderson Cancer Center (Houston, Texas) developed five novel human anti-IL13Rα2 antibodies (KLG-1 to KLG-5). The team evaluated the binding properties and target specificity of these antibodies in vitro, and performed in vivo 89Zr-immuno-PET imaging in a glioblastoma mouse model. Following the selection of KLG-3 as the most promising candidate, the researchers conducted a mass dose titration study. Ex vivo biodistribution analysis was used to determine the effective dosimetry of 177Lu-labeled KLG-3 therapy. The effectiveness of KLG-3 targeting was also tested in a melanoma mouse model.
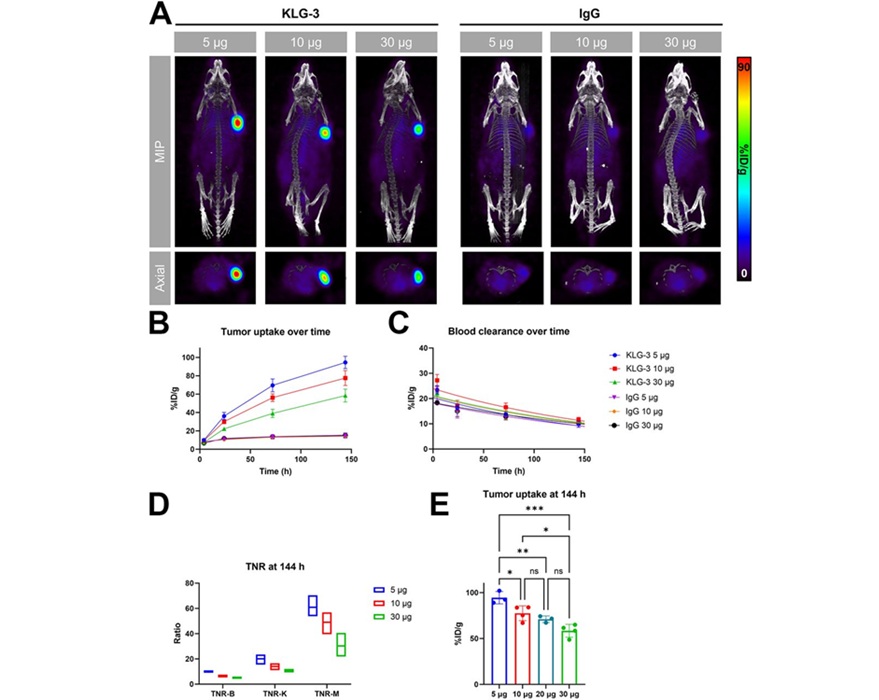
The lead antibody, KLG-3, demonstrated highly specific binding to targets in both human glioblastoma and melanoma models, producing high-contrast PET images with minimal off-target accumulation in healthy tissues. Prospective dosimetry of the 177Lu-labeled KLG-3 indicated therapeutic potential at relatively low injected doses, providing strong evidence to support the continued development of KLG-3 for use in radioimmunotherapy.
“These results demonstrate a significant advance in the use of IL13Rα2 as a viable target in cancer therapy. Implementation of the targeting moieties developed in this study may lead to highly specific and efficacious tumor-targeted drugs with little side effects to the patients,” said Simone Krebs, MD, associate professor at the University of Texas MD Anderson Cancer Center. “IL13Rα2 is known to be a harbinger of immunosuppression, and thus, IL13Rα2-targeted Immuno-PET may identify this molecular phenotype, which could aid in patient selection and an understanding of who may benefit from combinatorial strategies.”




 Guided Devices.jpg)









