First Digital Pathology Solution Receives FDA Clearance
|
By MedImaging International staff writers Posted on 25 Apr 2017 |
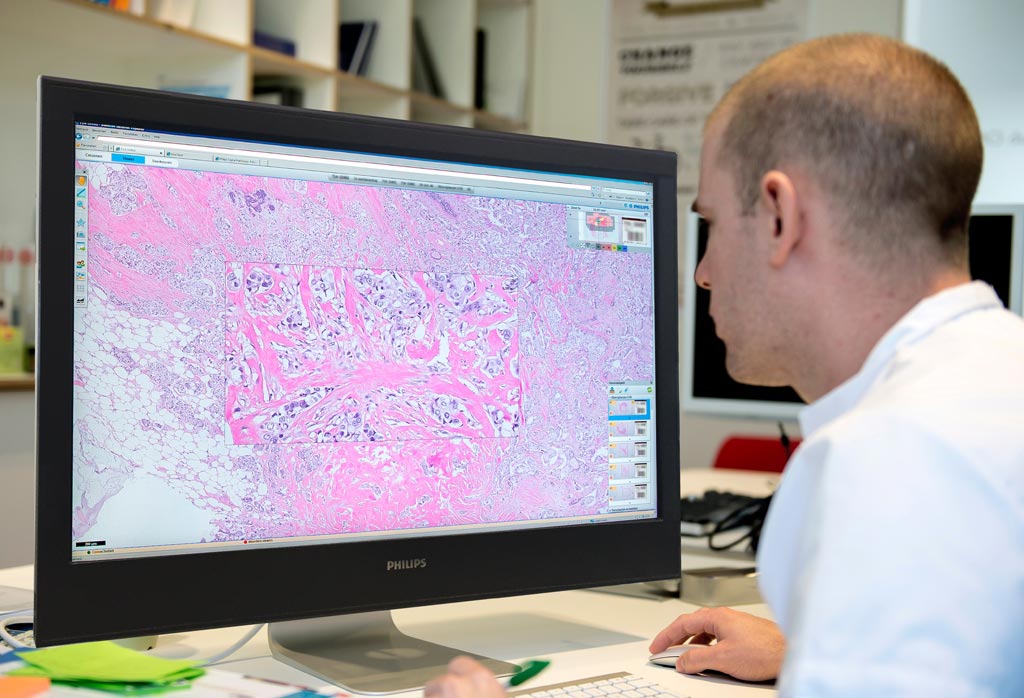
Image: A pathologist using the new digital pathology solution (Photo courtesy of Royal Philips).
After evaluating data from a clinical study including around 2,000 surgical pathology cases, the US FDA has cleared the first digital pathology solution for primary diagnostic use in the U.S.
Digital pathology can help pathologists view and diagnose digital images of surgical pathology slides, streamline workflows in pathology departments, and increase collaboration and diagnostic confidence.
The US Food and Drug Administration (FDA) approval will now enable Royal Philips to market its IntelliSite Pathology Solution for the digital pathology lab.
As cancer cases increase, pathology services need to deal with an increasing number and complexity of tests for cancer care. Digital pathology has already helped increase efficiency in the primary diagnosis of histopathology images outside the U.S. since 2014.
The Philips IntelliSite Pathology solution automates the creation, viewing and management of digital pathology images and includes advanced software tools that help manage information sharing, and other services. Digital pathology can also use computational pathology tools that provide more accurate image analysis, and predictive health management.
General Manager of Philips Digital Pathology Solutions, Russ Granzow, said, “Not only will it promote increased efficiencies and collaboration between pathologists, but it also opens a complete new dimension towards computational pathology which aims to increase accuracies and ultimately enhance patient care. The clearance for Philips IntelliSite Pathology Solution marks a major milestone for innovation in pathology services in the U.S.”
Digital pathology can help pathologists view and diagnose digital images of surgical pathology slides, streamline workflows in pathology departments, and increase collaboration and diagnostic confidence.
The US Food and Drug Administration (FDA) approval will now enable Royal Philips to market its IntelliSite Pathology Solution for the digital pathology lab.
As cancer cases increase, pathology services need to deal with an increasing number and complexity of tests for cancer care. Digital pathology has already helped increase efficiency in the primary diagnosis of histopathology images outside the U.S. since 2014.
The Philips IntelliSite Pathology solution automates the creation, viewing and management of digital pathology images and includes advanced software tools that help manage information sharing, and other services. Digital pathology can also use computational pathology tools that provide more accurate image analysis, and predictive health management.
General Manager of Philips Digital Pathology Solutions, Russ Granzow, said, “Not only will it promote increased efficiencies and collaboration between pathologists, but it also opens a complete new dimension towards computational pathology which aims to increase accuracies and ultimately enhance patient care. The clearance for Philips IntelliSite Pathology Solution marks a major milestone for innovation in pathology services in the U.S.”
Latest Imaging IT News
- New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
- Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
- AI-Based Mammography Triage Software Helps Dramatically Improve Interpretation Process
- Artificial Intelligence (AI) Program Accurately Predicts Lung Cancer Risk from CT Images
- Image Management Platform Streamlines Treatment Plans
- AI-Based Technology for Ultrasound Image Analysis Receives FDA Approval
- AI Technology for Detecting Breast Cancer Receives CE Mark Approval
- Digital Pathology Software Improves Workflow Efficiency
- Patient-Centric Portal Facilitates Direct Imaging Access
- New Workstation Supports Customer-Driven Imaging Workflow
Channels
Radiography
view channel
Novel Breast Imaging System Proves As Effective As Mammography
Breast cancer remains the most frequently diagnosed cancer among women. It is projected that one in eight women will be diagnosed with breast cancer during her lifetime, and one in 42 women who turn 50... Read more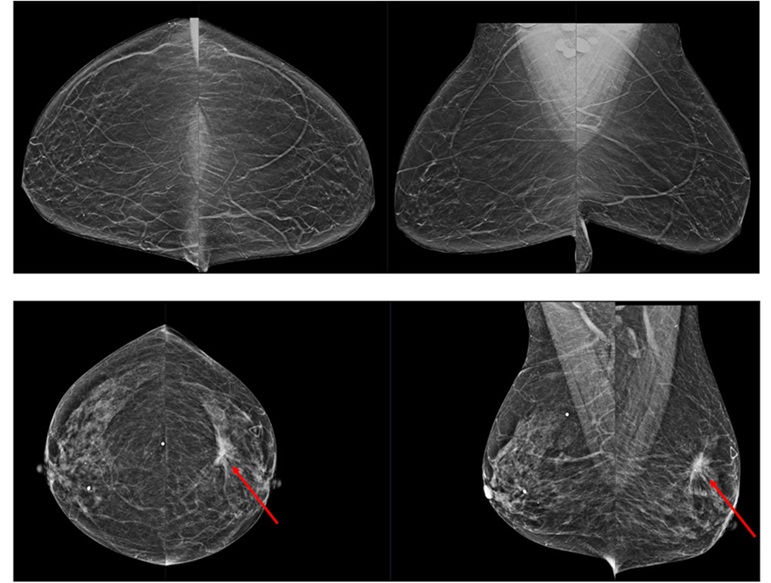
AI Assistance Improves Breast-Cancer Screening by Reducing False Positives
Radiologists typically detect one case of cancer for every 200 mammograms reviewed. However, these evaluations often result in false positives, leading to unnecessary patient recalls for additional testing,... Read moreMRI
view channel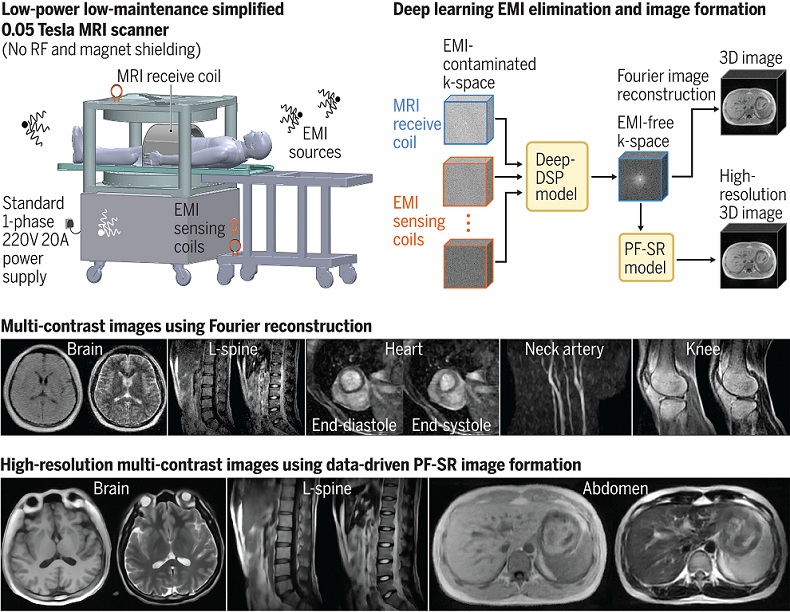
Low-Cost Whole-Body MRI Device Combined with AI Generates High-Quality Results
Magnetic Resonance Imaging (MRI) has significantly transformed healthcare, providing a noninvasive, radiation-free method for detailed imaging. It is especially promising for the future of medical diagnosis... Read more
World's First Whole-Body Ultra-High Field MRI Officially Comes To Market
The world's first whole-body ultra-high field (UHF) MRI has officially come to market, marking a remarkable advancement in diagnostic radiology. United Imaging (Shanghai, China) has secured clearance from the U.... Read moreUltrasound
view channel.jpg)
Diagnostic System Automatically Analyzes TTE Images to Identify Congenital Heart Disease
Congenital heart disease (CHD) is one of the most prevalent congenital anomalies worldwide, presenting substantial health and financial challenges for affected patients. Early detection and treatment of... Read more
Super-Resolution Imaging Technique Could Improve Evaluation of Cardiac Conditions
The heart depends on efficient blood circulation to pump blood throughout the body, delivering oxygen to tissues and removing carbon dioxide and waste. Yet, when heart vessels are damaged, it can disrupt... Read more
First AI-Powered POC Ultrasound Diagnostic Solution Helps Prioritize Cases Based On Severity
Ultrasound scans are essential for identifying and diagnosing various medical conditions, but often, patients must wait weeks or months for results due to a shortage of qualified medical professionals... Read moreNuclear Medicine
view channelNew PET Agent Rapidly and Accurately Visualizes Lesions in Clear Cell Renal Cell Carcinoma Patients
Clear cell renal cell cancer (ccRCC) represents 70-80% of renal cell carcinoma cases. While localized disease can be effectively treated with surgery and ablative therapies, one-third of patients either... Read more
New Imaging Technique Monitors Inflammation Disorders without Radiation Exposure
Imaging inflammation using traditional radiological techniques presents significant challenges, including radiation exposure, poor image quality, high costs, and invasive procedures. Now, new contrast... Read more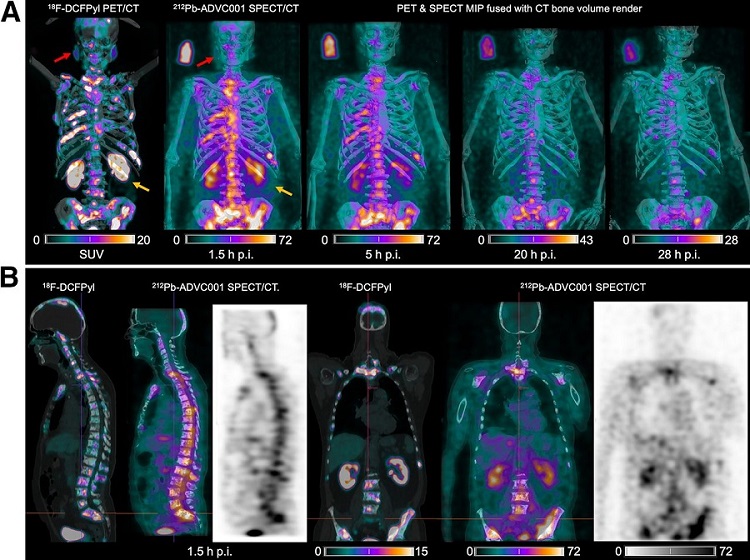
New SPECT/CT Technique Could Change Imaging Practices and Increase Patient Access
The development of lead-212 (212Pb)-PSMA–based targeted alpha therapy (TAT) is garnering significant interest in treating patients with metastatic castration-resistant prostate cancer. The imaging of 212Pb,... Read moreGeneral/Advanced Imaging
view channelBone Density Test Uses Existing CT Images to Predict Fractures
Osteoporotic fractures are not only devastating and deadly, especially hip fractures, but also impose significant costs. They rank among the top chronic diseases in terms of disability-adjusted life years... Read more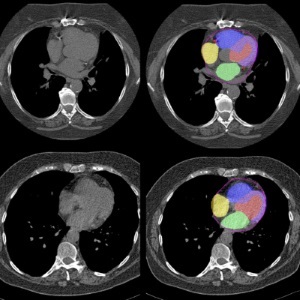
AI Predicts Cardiac Risk and Mortality from Routine Chest CT Scans
Heart disease remains the leading cause of death and is largely preventable, yet many individuals are unaware of their risk until it becomes severe. Early detection through screening can reveal heart issues,... Read moreIndustry News
view channel
Hologic Acquires UK-Based Breast Surgical Guidance Company Endomagnetics Ltd.
Hologic, Inc. (Marlborough, MA, USA) has entered into a definitive agreement to acquire Endomagnetics Ltd. (Cambridge, UK), a privately held developer of breast cancer surgery technologies, for approximately... Read more
Bayer and Google Partner on New AI Product for Radiologists
Medical imaging data comprises around 90% of all healthcare data, and it is a highly complex and rich clinical data modality and serves as a vital tool for diagnosing patients. Each year, billions of medical... Read more