US FDA Clears Smart Mobile-Connected High-Resolution Bladder Scanner
|
By MedImaging International staff writers Posted on 18 May 2016 |
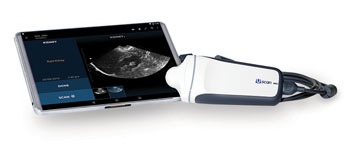
Image: The Uscan smart mobile-connected high-resolution bladder scanner is now cleared by the US FDA (Photo courtesy of Signostics).
A global medical device manufacturer has received US FDA clearance for a new urologic visualization device that can improve point-of-care clinical decision-making.
The device is the first mobile-connected ultrasound visualization device targeted at urologic care to receive US Food and Drug Administration (FDA) 510(k) clearance.
The Uscan device developed by Signostics (Bothell, WA, USA) has a removable probe, a high-resolution tablet with a touch screen, and handheld displays. The Uscan is compatible with the Android operating system, features built-in WiFi and Bluetooth connectivity, and is interoperable with Electronic Health Record (EHR) systems. The device does not require annual calibration, and provides real-time user guidance.
The Uscan can acquire up to 256 bladder slices, and uses algorithms to actively recognize bladder contours in 3D providing accurate volume measurements. The device can also be used for real-time ultrasound imaging of the bladder stones, gallbladder, prostate, pelvic floor, kidneys, for the placement of catheters, and for rapid visual tracking, and observation. The device can also be used in emergency departments, maternity wards, for pediatrics, oncology, rehabilitation, in home nursing, and in geriatrics.
CEO of Signostics, Kevin Goodwin, said, “Uscan doesn’t just scan; it sees – providing intelligent urologic visualization by leveraging science from current-day computer vision algorithms aimed at more efficient and confident point-of-care clinical decision-making. Uscan will exceed historical industry standards for bladder volume measurement accuracy yet will also enable use for other urologic imaging needs, reducing the delays and expense of engaging specialized ultrasound equipment or sonographers.”
Related Links:
Signostics
The device is the first mobile-connected ultrasound visualization device targeted at urologic care to receive US Food and Drug Administration (FDA) 510(k) clearance.
The Uscan device developed by Signostics (Bothell, WA, USA) has a removable probe, a high-resolution tablet with a touch screen, and handheld displays. The Uscan is compatible with the Android operating system, features built-in WiFi and Bluetooth connectivity, and is interoperable with Electronic Health Record (EHR) systems. The device does not require annual calibration, and provides real-time user guidance.
The Uscan can acquire up to 256 bladder slices, and uses algorithms to actively recognize bladder contours in 3D providing accurate volume measurements. The device can also be used for real-time ultrasound imaging of the bladder stones, gallbladder, prostate, pelvic floor, kidneys, for the placement of catheters, and for rapid visual tracking, and observation. The device can also be used in emergency departments, maternity wards, for pediatrics, oncology, rehabilitation, in home nursing, and in geriatrics.
CEO of Signostics, Kevin Goodwin, said, “Uscan doesn’t just scan; it sees – providing intelligent urologic visualization by leveraging science from current-day computer vision algorithms aimed at more efficient and confident point-of-care clinical decision-making. Uscan will exceed historical industry standards for bladder volume measurement accuracy yet will also enable use for other urologic imaging needs, reducing the delays and expense of engaging specialized ultrasound equipment or sonographers.”
Related Links:
Signostics
Latest Imaging IT News
- New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
- Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
- AI-Based Mammography Triage Software Helps Dramatically Improve Interpretation Process
- Artificial Intelligence (AI) Program Accurately Predicts Lung Cancer Risk from CT Images
- Image Management Platform Streamlines Treatment Plans
- AI-Based Technology for Ultrasound Image Analysis Receives FDA Approval
- AI Technology for Detecting Breast Cancer Receives CE Mark Approval
- Digital Pathology Software Improves Workflow Efficiency
- Patient-Centric Portal Facilitates Direct Imaging Access
- New Workstation Supports Customer-Driven Imaging Workflow
Channels
Radiography
view channel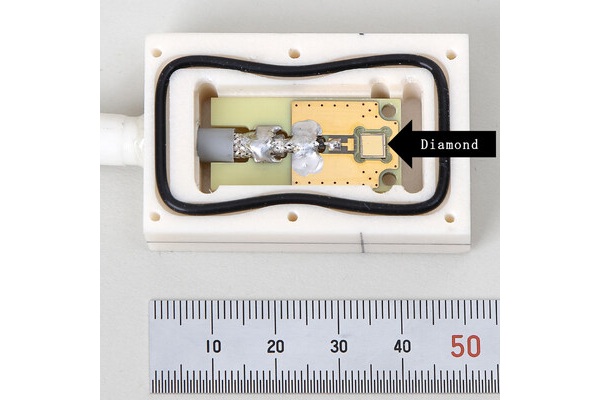
World's Largest Class Single Crystal Diamond Radiation Detector Opens New Possibilities for Diagnostic Imaging
Diamonds possess ideal physical properties for radiation detection, such as exceptional thermal and chemical stability along with a quick response time. Made of carbon with an atomic number of six, diamonds... Read more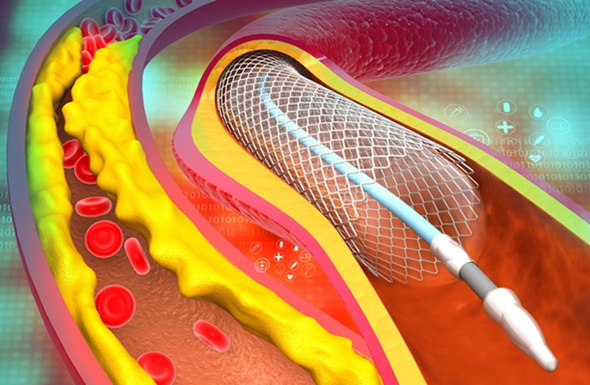
AI-Powered Imaging Technique Shows Promise in Evaluating Patients for PCI
Percutaneous coronary intervention (PCI), also known as coronary angioplasty, is a minimally invasive procedure where small metal tubes called stents are inserted into partially blocked coronary arteries... Read moreMRI
view channel
AI Tool Predicts Relapse of Pediatric Brain Cancer from Brain MRI Scans
Many pediatric gliomas are treatable with surgery alone, but relapses can be catastrophic. Predicting which patients are at risk for recurrence remains challenging, leading to frequent follow-ups with... Read more
AI Tool Tracks Effectiveness of Multiple Sclerosis Treatments Using Brain MRI Scans
Multiple sclerosis (MS) is a condition in which the immune system attacks the brain and spinal cord, leading to impairments in movement, sensation, and cognition. Magnetic Resonance Imaging (MRI) markers... Read more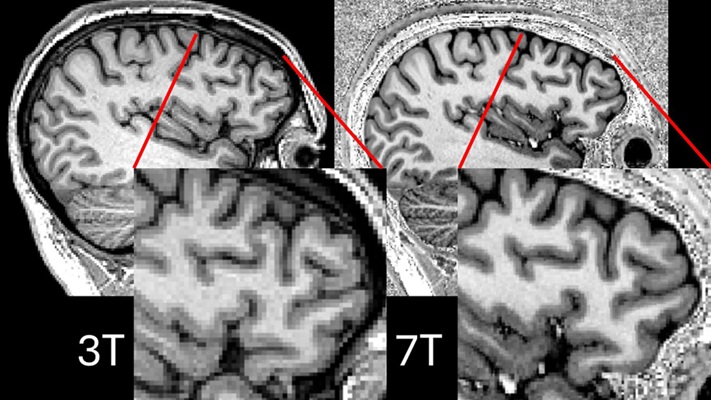
Ultra-Powerful MRI Scans Enable Life-Changing Surgery in Treatment-Resistant Epileptic Patients
Approximately 360,000 individuals in the UK suffer from focal epilepsy, a condition in which seizures spread from one part of the brain. Around a third of these patients experience persistent seizures... Read moreNuclear Medicine
view channel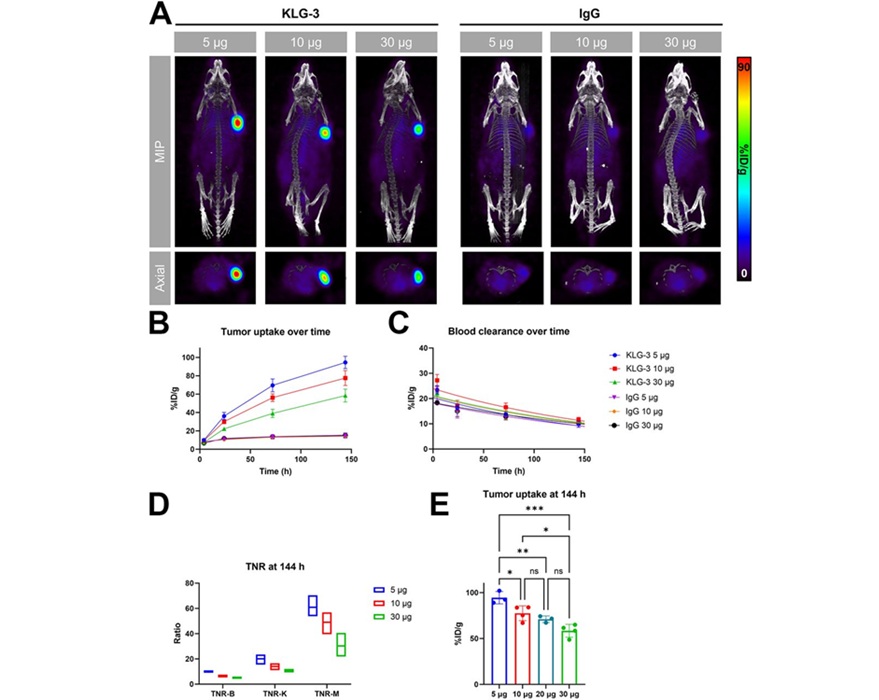
Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
Interleukin-13 receptor α-2 (IL13Rα2) is a cell surface receptor commonly found in solid tumors such as glioblastoma, melanoma, and breast cancer. It is minimally expressed in normal tissues, making it... Read more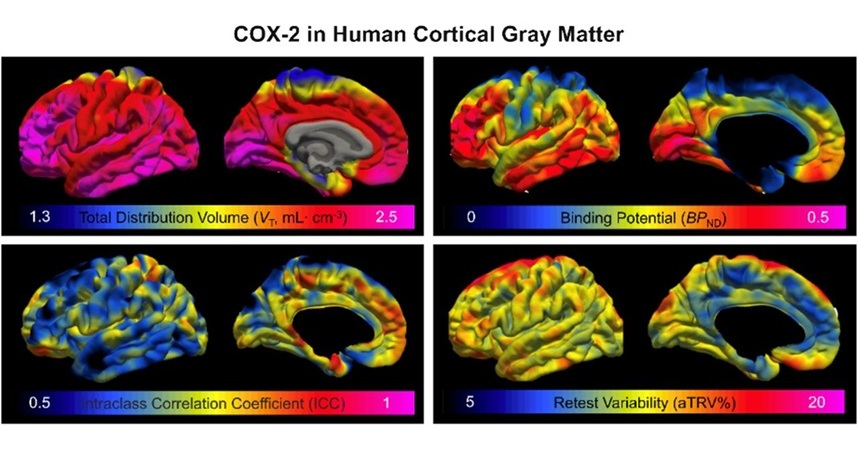
Novel PET Imaging Approach Offers Never-Before-Seen View of Neuroinflammation
COX-2, an enzyme that plays a key role in brain inflammation, can be significantly upregulated by inflammatory stimuli and neuroexcitation. Researchers suggest that COX-2 density in the brain could serve... Read moreGeneral/Advanced Imaging
view channel
AI-Powered Imaging System Improves Lung Cancer Diagnosis
Given the need to detect lung cancer at earlier stages, there is an increasing need for a definitive diagnostic pathway for patients with suspicious pulmonary nodules. However, obtaining tissue samples... Read more
AI Model Significantly Enhances Low-Dose CT Capabilities
Lung cancer remains one of the most challenging diseases, making early diagnosis vital for effective treatment. Fortunately, advancements in artificial intelligence (AI) are revolutionizing lung cancer... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel
GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
GE HealthCare (Chicago, IL, USA) has entered into a collaboration with NVIDIA (Santa Clara, CA, USA), expanding the existing relationship between the two companies to focus on pioneering innovation in... Read more
Patient-Specific 3D-Printed Phantoms Transform CT Imaging
New research has highlighted how anatomically precise, patient-specific 3D-printed phantoms are proving to be scalable, cost-effective, and efficient tools in the development of new CT scan algorithms... Read more
Siemens and Sectra Collaborate on Enhancing Radiology Workflows
Siemens Healthineers (Forchheim, Germany) and Sectra (Linköping, Sweden) have entered into a collaboration aimed at enhancing radiologists' diagnostic capabilities and, in turn, improving patient care... Read more