Imaging Informatics Provider Announces FDA Clearance of New Platform
|
By MedImaging International staff writers Posted on 08 Sep 2015 |
A new clinical decision support platform for enterprise Picture Archiving and Communication System (PACS) and Vendor Neutral Archives (VNA) has received US Food and Drug Administration (FDA) 510 (k) clearance.
The platform was designed to integrate and align with enterprise PACS and VNA systems using industry-standard formats such as the US American College of Radiology (ACR) Imaging 3.0 initiative.
The platform was developed by HealthMyne (Madison, WI, USA), an imaging informatics company, and is intended to help radiologists provide diagnostic imaging data to clinicians before and after performing an imaging exam during the clinical workflow.
HealthMyne’s image data mining platforms can help radiologist and oncologists increase their workflow efficiency by automating data capture, and structured reports. The platform will provide integrated patient progression timelines, and analytics and enable clinicians to compare data for an individual patient with a patient cohort. HealthMyne’s analytic tools are also intended to help researchers explore quantitative imaging biomarkers, and support improved clinical trials of innovative therapies.
Praveen Sinha, CEO of HealthMyne, said, “Having a strong diagnostic imaging platform cleared by the FDA is the first step in our vision of bringing imaging informatics to mainstream healthcare. Significant achievements are being realized elsewhere through clinical data aggregation and analysis, yet imaging has gone largely untapped. As an example, the potential impact of streamlined patient cohort comparison on personalized medicine is truly exciting.”
Related Links:
HealthMyne
The platform was designed to integrate and align with enterprise PACS and VNA systems using industry-standard formats such as the US American College of Radiology (ACR) Imaging 3.0 initiative.
The platform was developed by HealthMyne (Madison, WI, USA), an imaging informatics company, and is intended to help radiologists provide diagnostic imaging data to clinicians before and after performing an imaging exam during the clinical workflow.
HealthMyne’s image data mining platforms can help radiologist and oncologists increase their workflow efficiency by automating data capture, and structured reports. The platform will provide integrated patient progression timelines, and analytics and enable clinicians to compare data for an individual patient with a patient cohort. HealthMyne’s analytic tools are also intended to help researchers explore quantitative imaging biomarkers, and support improved clinical trials of innovative therapies.
Praveen Sinha, CEO of HealthMyne, said, “Having a strong diagnostic imaging platform cleared by the FDA is the first step in our vision of bringing imaging informatics to mainstream healthcare. Significant achievements are being realized elsewhere through clinical data aggregation and analysis, yet imaging has gone largely untapped. As an example, the potential impact of streamlined patient cohort comparison on personalized medicine is truly exciting.”
Related Links:
HealthMyne
Latest Imaging IT News
- New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
- Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
- AI-Based Mammography Triage Software Helps Dramatically Improve Interpretation Process
- Artificial Intelligence (AI) Program Accurately Predicts Lung Cancer Risk from CT Images
- Image Management Platform Streamlines Treatment Plans
- AI-Based Technology for Ultrasound Image Analysis Receives FDA Approval
- AI Technology for Detecting Breast Cancer Receives CE Mark Approval
- Digital Pathology Software Improves Workflow Efficiency
- Patient-Centric Portal Facilitates Direct Imaging Access
- New Workstation Supports Customer-Driven Imaging Workflow
Channels
Radiography
view channel
Routine Mammograms Could Predict Future Cardiovascular Disease in Women
Mammograms are widely used to screen for breast cancer, but they may also contain overlooked clues about cardiovascular health. Calcium deposits in the arteries of the breast signal stiffening blood vessels,... Read more
AI Detects Early Signs of Aging from Chest X-Rays
Chronological age does not always reflect how fast the body is truly aging, and current biological age tests often rely on DNA-based markers that may miss early organ-level decline. Detecting subtle, age-related... Read moreMRI
view channel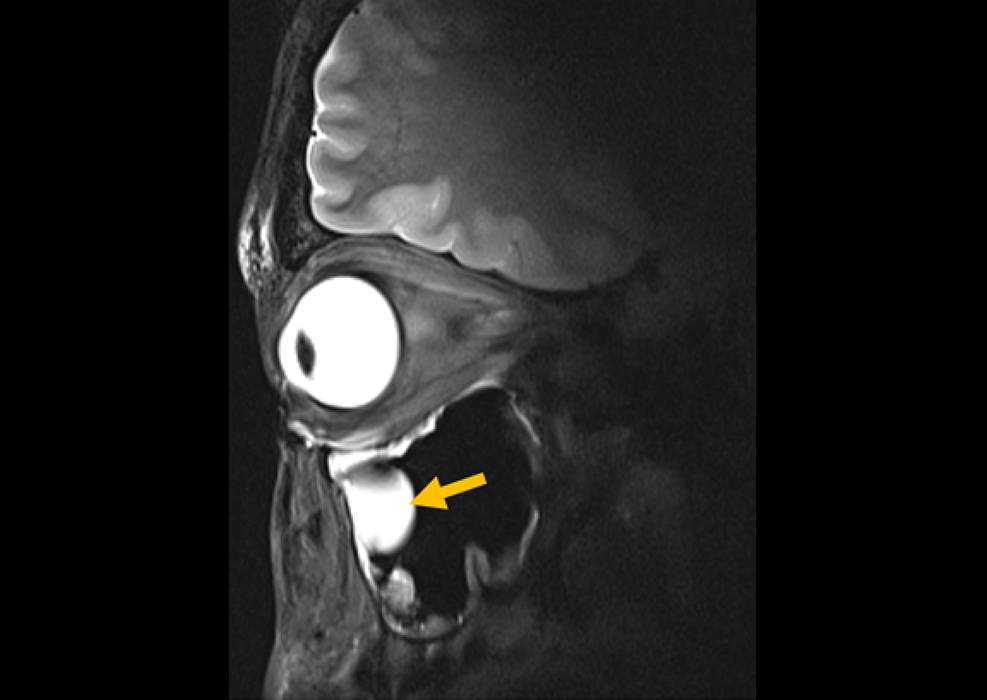
New Material Boosts MRI Image Quality
Magnetic resonance imaging (MRI) is a cornerstone of modern diagnostics, yet certain deep or anatomically complex tissues, including delicate structures of the eye and orbit, remain difficult to visualize clearly.... Read more
AI Model Reads and Diagnoses Brain MRI in Seconds
Brain MRI scans are critical for diagnosing strokes, hemorrhages, and other neurological disorders, but interpreting them can take hours or even days due to growing demand and limited specialist availability.... Read moreMRI Scan Breakthrough to Help Avoid Risky Invasive Tests for Heart Patients
Heart failure patients often require right heart catheterization to assess how severely their heart is struggling to pump blood, a procedure that involves inserting a tube into the heart to measure blood... Read more
MRI Scans Reveal Signature Patterns of Brain Activity to Predict Recovery from TBI
Recovery after traumatic brain injury (TBI) varies widely, with some patients regaining full function while others are left with lasting disabilities. Prognosis is especially difficult to assess in patients... Read moreUltrasound
view channel
Reusable Gel Pad Made from Tamarind Seed Could Transform Ultrasound Examinations
Ultrasound imaging depends on a conductive gel to eliminate air between the probe and the skin so sound waves can pass clearly into the body. While the imaging technology is fast, safe, and noninvasive,... Read more
AI Model Accurately Detects Placenta Accreta in Pregnancy Before Delivery
Placenta accreta spectrum (PAS) is a life-threatening pregnancy complication in which the placenta abnormally attaches to the uterine wall. The condition is a leading cause of maternal mortality and morbidity... Read moreNuclear Medicine
view channel
Radiopharmaceutical Molecule Marker to Improve Choice of Bladder Cancer Therapies
Targeted cancer therapies only work when tumor cells express the specific molecular structures they are designed to attack. In urothelial carcinoma, a common form of bladder cancer, the cell surface protein... Read more
Cancer “Flashlight” Shows Who Can Benefit from Targeted Treatments
Targeted cancer therapies can be highly effective, but only when a patient’s tumor expresses the specific protein the treatment is designed to attack. Determining this usually requires biopsies or advanced... Read moreGeneral/Advanced Imaging
view channel
AI Tool Offers Prognosis for Patients with Head and Neck Cancer
Oropharyngeal cancer is a form of head and neck cancer that can spread through lymph nodes, significantly affecting survival and treatment decisions. Current therapies often involve combinations of surgery,... Read more
New 3D Imaging System Addresses MRI, CT and Ultrasound Limitations
Medical imaging is central to diagnosing and managing injuries, cancer, infections, and chronic diseases, yet existing tools each come with trade-offs. Ultrasound, X-ray, CT, and MRI can be costly, time-consuming,... Read moreIndustry News
view channel
Nuclear Medicine Set for Continued Growth Driven by Demand for Precision Diagnostics
Clinical imaging services face rising demand for precise molecular diagnostics and targeted radiopharmaceutical therapy as cancer and chronic disease rates climb. A new market analysis projects rapid expansion... Read more