Unique Innovative in-Procedure Ablation Confirmation Software Receives FDA Clearance
|
By MedImaging International staff writers Posted on 08 Sep 2015 |
The first and only in-procedure ablation confirmation software that is part of an ablation system, and is intended for the ablation of soft tissue lesions, has received clearance from the US Food and Drug Administration (FDA; Silver Spring, MD USA).
The software was developed by a privately held medical device company and is intended for use with their ablation system. The software uses images from Computed Tomography (CT) scanners and Picture Archiving and Communication Systems (PACS) and helps clinicians identify ablation targets, assess correct placement of ablation probes, and confirm post procedure ablation zones.
The Ablation Confirmation (AC) software, user interface, and NewWave Intelligent Ablation system were developed by NewWave Medical (Madison WI, USA). The AC software imports images from a CT scanner and PACS, and displays them on a dedicated monitor on the NewWave Intelligent Ablation system.
Dan Sullivan, CEO NeuWave Medical, said, “This is a significant step forward, as today physicians performing an ablation have to view patient CT scans with the naked eye on side by side monitors outside the procedure room. Now, with Ablation Confirmation, only available from NeuWave, there is no need to imagine what the ablation scans look like. The system will overlay pre‐ and post‐ablation scans to show the physician whether or not the ablation is complete, all while never leaving the procedure room. This is game changing for physicians and expected to set a new standard of care for the ablation of soft tissue lesions.”
Related Links:
NewWave Medical
The software was developed by a privately held medical device company and is intended for use with their ablation system. The software uses images from Computed Tomography (CT) scanners and Picture Archiving and Communication Systems (PACS) and helps clinicians identify ablation targets, assess correct placement of ablation probes, and confirm post procedure ablation zones.
The Ablation Confirmation (AC) software, user interface, and NewWave Intelligent Ablation system were developed by NewWave Medical (Madison WI, USA). The AC software imports images from a CT scanner and PACS, and displays them on a dedicated monitor on the NewWave Intelligent Ablation system.
Dan Sullivan, CEO NeuWave Medical, said, “This is a significant step forward, as today physicians performing an ablation have to view patient CT scans with the naked eye on side by side monitors outside the procedure room. Now, with Ablation Confirmation, only available from NeuWave, there is no need to imagine what the ablation scans look like. The system will overlay pre‐ and post‐ablation scans to show the physician whether or not the ablation is complete, all while never leaving the procedure room. This is game changing for physicians and expected to set a new standard of care for the ablation of soft tissue lesions.”
Related Links:
NewWave Medical
Latest Imaging IT News
- New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
- Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
- AI-Based Mammography Triage Software Helps Dramatically Improve Interpretation Process
- Artificial Intelligence (AI) Program Accurately Predicts Lung Cancer Risk from CT Images
- Image Management Platform Streamlines Treatment Plans
- AI-Based Technology for Ultrasound Image Analysis Receives FDA Approval
- AI Technology for Detecting Breast Cancer Receives CE Mark Approval
- Digital Pathology Software Improves Workflow Efficiency
- Patient-Centric Portal Facilitates Direct Imaging Access
- New Workstation Supports Customer-Driven Imaging Workflow
Channels
Radiography
view channel
Novel Breast Imaging System Proves As Effective As Mammography
Breast cancer remains the most frequently diagnosed cancer among women. It is projected that one in eight women will be diagnosed with breast cancer during her lifetime, and one in 42 women who turn 50... Read more
AI Assistance Improves Breast-Cancer Screening by Reducing False Positives
Radiologists typically detect one case of cancer for every 200 mammograms reviewed. However, these evaluations often result in false positives, leading to unnecessary patient recalls for additional testing,... Read moreMRI
view channel
World's First Whole-Body Ultra-High Field MRI Officially Comes To Market
The world's first whole-body ultra-high field (UHF) MRI has officially come to market, marking a remarkable advancement in diagnostic radiology. United Imaging (Shanghai, China) has secured clearance from the U.... Read more
World's First Sensor Detects Errors in MRI Scans Using Laser Light and Gas
MRI scanners are daily tools for doctors and healthcare professionals, providing unparalleled 3D imaging of the brain, vital organs, and soft tissues, far surpassing other imaging technologies in quality.... Read more
Diamond Dust Could Offer New Contrast Agent Option for Future MRI Scans
Gadolinium, a heavy metal used for over three decades as a contrast agent in medical imaging, enhances the clarity of MRI scans by highlighting affected areas. Despite its utility, gadolinium not only... Read more.jpg)
Combining MRI with PSA Testing Improves Clinical Outcomes for Prostate Cancer Patients
Prostate cancer is a leading health concern globally, consistently being one of the most common types of cancer among men and a major cause of cancer-related deaths. In the United States, it is the most... Read moreUltrasound
view channel
First AI-Powered POC Ultrasound Diagnostic Solution Helps Prioritize Cases Based On Severity
Ultrasound scans are essential for identifying and diagnosing various medical conditions, but often, patients must wait weeks or months for results due to a shortage of qualified medical professionals... Read more
Largest Model Trained On Echocardiography Images Assesses Heart Structure and Function
Foundation models represent an exciting frontier in generative artificial intelligence (AI), yet many lack the specialized medical data needed to make them applicable in healthcare settings.... Read more.jpg)
Groundbreaking Technology Enables Precise, Automatic Measurement of Peripheral Blood Vessels
The current standard of care of using angiographic information is often inadequate for accurately assessing vessel size in the estimated 20 million people in the U.S. who suffer from peripheral vascular disease.... Read moreNuclear Medicine
view channelNew PET Agent Rapidly and Accurately Visualizes Lesions in Clear Cell Renal Cell Carcinoma Patients
Clear cell renal cell cancer (ccRCC) represents 70-80% of renal cell carcinoma cases. While localized disease can be effectively treated with surgery and ablative therapies, one-third of patients either... Read more
New Imaging Technique Monitors Inflammation Disorders without Radiation Exposure
Imaging inflammation using traditional radiological techniques presents significant challenges, including radiation exposure, poor image quality, high costs, and invasive procedures. Now, new contrast... Read more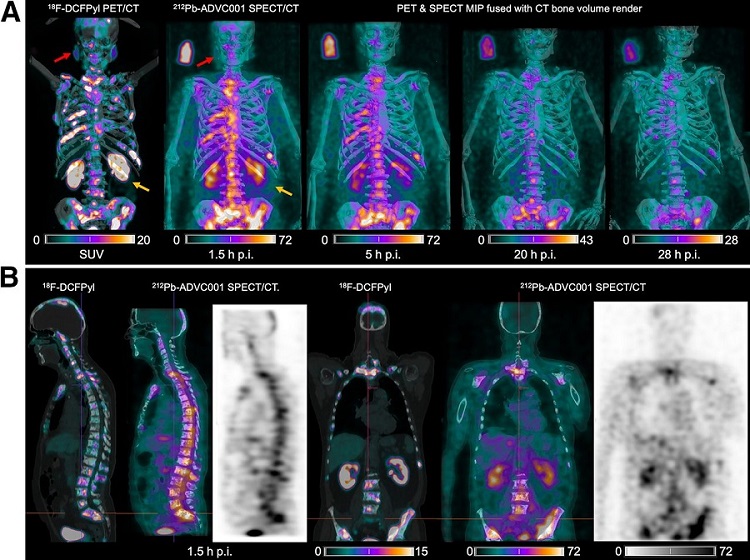
New SPECT/CT Technique Could Change Imaging Practices and Increase Patient Access
The development of lead-212 (212Pb)-PSMA–based targeted alpha therapy (TAT) is garnering significant interest in treating patients with metastatic castration-resistant prostate cancer. The imaging of 212Pb,... Read moreGeneral/Advanced Imaging
view channel
Radiation Therapy Computed Tomography Solution Boosts Imaging Accuracy
One of the most significant challenges in oncology care is disease complexity in terms of the variety of cancer types and the individualized presentation of each patient. This complexity necessitates a... Read more
PET Scans Reveal Hidden Inflammation in Multiple Sclerosis Patients
A key challenge for clinicians treating patients with multiple sclerosis (MS) is that after a certain amount of time, they continue to worsen even though their MRIs show no change. A new study has now... Read moreIndustry News
view channel
Hologic Acquires UK-Based Breast Surgical Guidance Company Endomagnetics Ltd.
Hologic, Inc. (Marlborough, MA, USA) has entered into a definitive agreement to acquire Endomagnetics Ltd. (Cambridge, UK), a privately held developer of breast cancer surgery technologies, for approximately... Read more
Bayer and Google Partner on New AI Product for Radiologists
Medical imaging data comprises around 90% of all healthcare data, and it is a highly complex and rich clinical data modality and serves as a vital tool for diagnosing patients. Each year, billions of medical... Read more