Vital Signs Monitor Overcomes the MRI Environment
|
By MedImaging International staff writers Posted on 24 Nov 2017 |
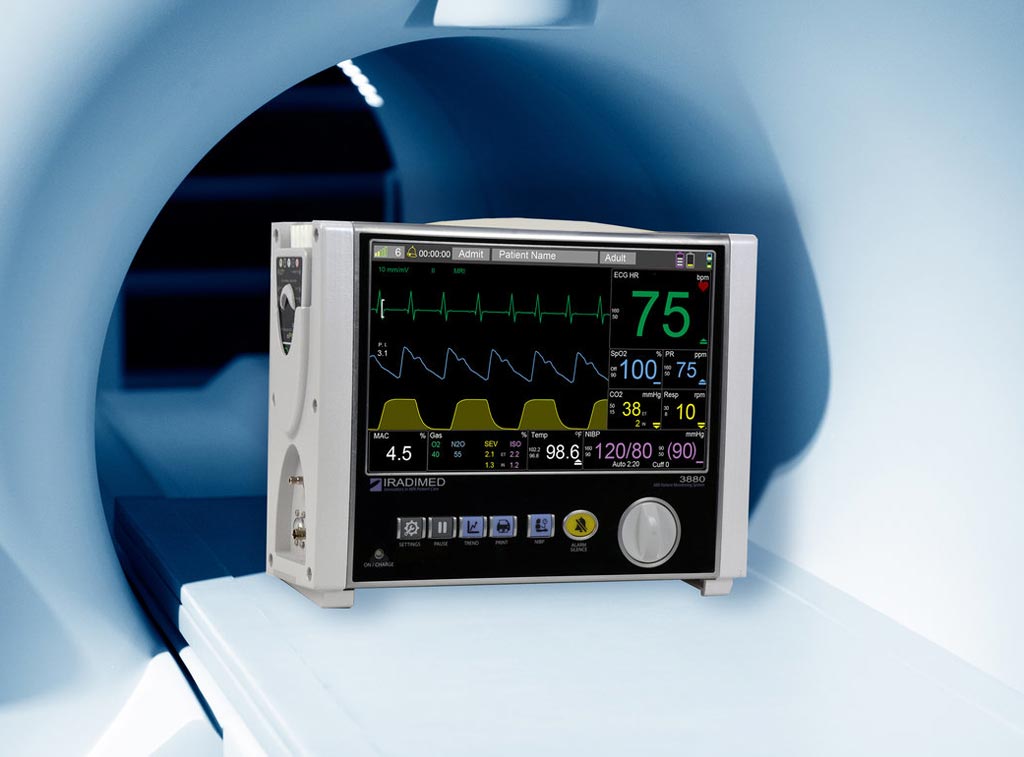
Image: The 3880 MRI compatible patient vital signs monitoring system (Photo courtesy of Iradimed).
An innovative magnetic resonance imaging (MRI) compatible vital signs monitor allows patients to be supervised during imaging.
The Iradimed (Winter Springs, FL, USA) 3880 MRI compatible patient monitoring system is a compact, lightweight device designed to facilitate transportation of patients from a critical care unit to the MRI scanner and back, increasing patient safety by ensuring uninterrupted vital signs monitoring and decreasing the amount of time critically ill patients are away from their care units. The system features a 30,000 gauss rating in a non-magnetic construction with a small form factor, which means it can operate virtually anywhere in the MRI scanner room.
An optional paired tablet allows for remote viewing of patient vital signs and control of the monitoring unit from within the MRI control room. Additional features include wireless electrocardiogram (ECG) with dynamic gradient filtering; wireless oxygen saturation (SpO2) using Masimo algorithms; respiratory CO2; non-invasive blood pressure and patient temperature measurement; and optional advanced multi-gas anesthetic agent unit featuring continuous minimum alveolar concentration measurements.
“The Iradimed 3880 monitor’s compact non-magnetic design positions Iradimed as the only company in the world with a MRI compatible patient monitor that can be used to transport patients from the critical care departments of hospitals to the MRI scanner room and back to critical care,” said Roger Susi, President and CEO of Iradimed. “It has an easy-to-use design and unique wireless tablet remote control that allows for the effective communication of vital signs information to clinicians.”
The MRI Compatible definition is typically applied to devices not considered to significantly affect the quality of the diagnostic information, nor have their operations affected by the MRI scanner. The MRI conditions in which the device was tested need to be specified, since a device which is safe under one set of conditions may not be found to be so under more extreme MRI conditions.
The Iradimed (Winter Springs, FL, USA) 3880 MRI compatible patient monitoring system is a compact, lightweight device designed to facilitate transportation of patients from a critical care unit to the MRI scanner and back, increasing patient safety by ensuring uninterrupted vital signs monitoring and decreasing the amount of time critically ill patients are away from their care units. The system features a 30,000 gauss rating in a non-magnetic construction with a small form factor, which means it can operate virtually anywhere in the MRI scanner room.
An optional paired tablet allows for remote viewing of patient vital signs and control of the monitoring unit from within the MRI control room. Additional features include wireless electrocardiogram (ECG) with dynamic gradient filtering; wireless oxygen saturation (SpO2) using Masimo algorithms; respiratory CO2; non-invasive blood pressure and patient temperature measurement; and optional advanced multi-gas anesthetic agent unit featuring continuous minimum alveolar concentration measurements.
“The Iradimed 3880 monitor’s compact non-magnetic design positions Iradimed as the only company in the world with a MRI compatible patient monitor that can be used to transport patients from the critical care departments of hospitals to the MRI scanner room and back to critical care,” said Roger Susi, President and CEO of Iradimed. “It has an easy-to-use design and unique wireless tablet remote control that allows for the effective communication of vital signs information to clinicians.”
The MRI Compatible definition is typically applied to devices not considered to significantly affect the quality of the diagnostic information, nor have their operations affected by the MRI scanner. The MRI conditions in which the device was tested need to be specified, since a device which is safe under one set of conditions may not be found to be so under more extreme MRI conditions.
Latest MRI News
- Groundbreaking AI-Powered Software Significantly Enhances Brain MRI
- MRI Predicts Patient Outcomes and Tumor Recurrence in Rectal Cancer Patients
- Portable MRI System Dramatically Cuts Time-To-Scan vs. Conventional MRI in Stroke Patients
- Novel Model Identifies Focal Cortical Dysplasia Lesion from MRI Scans
- AI-Enhanced MRI Improves Diagnosis of Brain Disorders
- New Cardiac MRI Strategy Guides Ablation Procedures for Complex Tachycardias
- AI Model Achieves Clinical Expert Level Accuracy in Analyzing Complex MRIs and 3D Medical Scans
- MRI Provides Early Warning System for Glioblastoma Growth
- AI Algorithm Analyzes MRI Scans to Determine Best Rectal Cancer Treatment Strategy
- AI Software Uses MRI Scans to Automatically Segment Key Brain Structures for Improved Radiation Therapy Planning
- AI Software Analyzes Neuroimaging Data and Patient Information to Diagnose 10 Types of Dementia
- Metamaterials to Make MRI Scans Faster, Cheaper, and More Accurate
- Deep Learning Enables Accurate, Automated Quality Control Image Assessment for Liver MR Elastography
- Deep Learning-Based AI for Prostate MRI Helps Improve Risk Assessment and Avoid Unnecessary Biopsies
- Breakthrough Heart MRI Technique Accurately Predicts Heart Failure Risk in General Population
- MRI Could Help Doctors Predict More Aggressive Prostate Cancer in Patients
Channels
Radiography
view channel
Novel Breast Cancer Screening Technology Could Offer Superior Alternative to Mammogram
Breast cancer represents 15.5% of new cancer cases and 7% of cancer-related deaths in the United States. Approximately 13.1% of women will be diagnosed with breast cancer during their lifetime.... Read more
Artificial Intelligence Accurately Predicts Breast Cancer Years Before Diagnosis
Mammography screening helps reduce breast cancer mortality; however, its accuracy is not perfect. For decades, various strategies have been employed to enhance the interpretive performance of mammography,... Read more (1).jpg)
AI-Powered Chest X-Ray Detects Pulmonary Nodules Three Years Before Lung Cancer Symptoms
Lung cancer has one of the worst survival rates among all cancers, with more than two-thirds of patients diagnosed at an advanced stage, making curative treatment no longer viable. The issue of missed... Read moreMRI
view channel
Groundbreaking AI-Powered Software Significantly Enhances Brain MRI
Contrast-enhanced magnetic resonance imaging (MRI) utilizes contrast agents to illuminate specific tissues or abnormalities, leading to improved visualization and more comprehensive information.... Read more.jpeg)
MRI Predicts Patient Outcomes and Tumor Recurrence in Rectal Cancer Patients
Colorectal cancer is on the rise among younger adults—those under 50—while it has been declining in older populations. It is estimated that approximately 1 in 23 men and 1 in 25 women will be diagnosed... Read more
Portable MRI System Dramatically Cuts Time-To-Scan vs. Conventional MRI in Stroke Patients
Imaging of the brain is crucial for the management of acute stroke and transient ischemic attack. Key roles of imaging include confirming the diagnosis, determining treatment eligibility, indicating the... Read moreUltrasound
view channel
Ultrasound Can Identify Sources of Brain-Related Issues and Disorders Before Treatment
For many years, healthcare professionals worldwide have relied on ultrasound to monitor the growth of unborn infants and evaluate the health of internal organs. However, ultrasound technology, once primarily... Read more
New Guideline on Handling Endobronchial Ultrasound Transbronchial Needle Samples
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has become the standard procedure for the initial diagnosis and staging of lung cancer; however, there is limited guidance on... Read more
Groundbreaking Ultrasound-Guided Needle Insertion System Improves Medical Procedures
Ultrasound-guided neuraxial procedures have traditionally been hampered by technical challenges, such as the need for three hands to manage the needle, probe, and syringe simultaneously, steep needle angles... Read moreNuclear Medicine
view channel
PET Software Enhances Diagnosis and Monitoring of Alzheimer's Disease
Alzheimer’s disease is marked by the buildup of beta-amyloid plaques and tau protein tangles in the brain. These deposits of beta-amyloid and tau appear in various brain regions at differing rates as the brain ages.... Read more.jpg)
New Photon-Counting CT Technique Diagnoses Osteoarthritis Before Symptoms Develop
X-ray imaging has evolved significantly since its introduction in 1895. This technique is known for capturing images quickly, making it ideal for emergency situations; however, it does not provide adequate... Read moreGeneral/Advanced Imaging
view channel
Low-Dose CT Screening for Lung Cancer Can Benefit Heavy Smokers
Lung cancer is often diagnosed at a late stage, with only about one-fifth to one-sixth of patients surviving five years after diagnosis. A new report now suggests that low-dose computed tomography (CT)... Read more![Image: A kidney showing positive [89Zr]Zr-girentuximab PET and histologically confirmed clear-cell renal cell carcinoma (Photo courtesy of Dr. Brian Shuch/UCLA Health) Image: A kidney showing positive [89Zr]Zr-girentuximab PET and histologically confirmed clear-cell renal cell carcinoma (Photo courtesy of Dr. Brian Shuch/UCLA Health)](https://globetechcdn.com/mobile_medicalimaging/images/stories/articles/article_images/2024-10-04/ca9scan.jpg)
Non-Invasive Imaging Technique Accurately Detects Aggressive Kidney Cancer
Kidney cancers, known as renal cell carcinomas, account for 90% of solid kidney tumors, with over 81,000 new cases diagnosed annually in the United States. Among the various types, clear-cell renal cell... Read more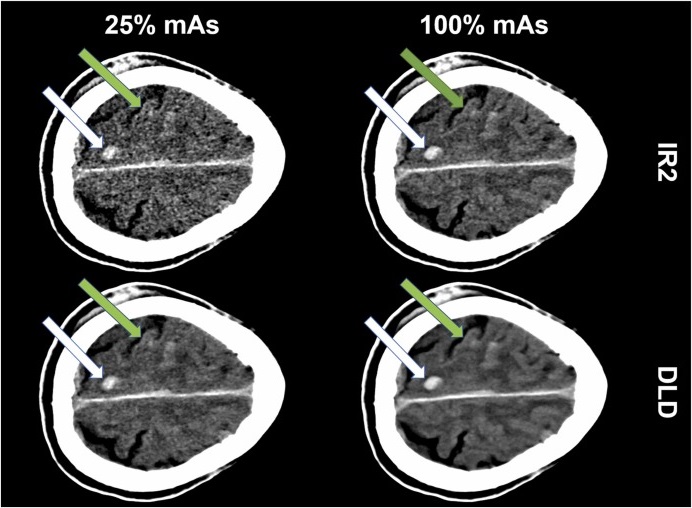
AI Algorithm Reduces Unnecessary Radiation Exposure in Traumatic Neuroradiological CT Scans
Traumatic neuroradiological emergencies encompass conditions that require immediate and accurate diagnosis for effective treatment and optimal patient outcomes. These emergencies can include injuries to... Read more
New Solution Enhances AI-Based Quality Control and Diagnosis in Medical Imaging
Medical image data makes up 80% of clinical data, and artificial intelligence (AI) offers significant potential to unlock its full value, which is crucial for clinical diagnosis, decision-making, and disease... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel.jpeg)
Philips and Medtronic Partner on Stroke Care
A stroke is typically an acute incident primarily caused by a blockage in a brain blood vessel, which disrupts the adequate blood supply to brain tissue and results in the permanent loss of brain cells.... Read more
Siemens and Medtronic Enter into Global Partnership for Advancing Spine Care Imaging Technologies
A new global partnership aims to explore opportunities to further expand access to advanced pre-and post-operative imaging technologies for spine care. Medtronic plc (Galway, Ireland) and Siemens Healthineers... Read more
RSNA 2024 Technical Exhibits to Showcase Latest Advances in Radiology
The Radiological Society of North America (RSNA, Oak Brook, IL, USA) has announced highlights of the Technical Exhibits at RSNA 2024: Building Intelligent Connections, the Society’s 110th Scientific Assembly... Read more












.jpg)