Groundbreaking AI-Powered Software Significantly Enhances Brain MRI
Posted on 25 Oct 2024
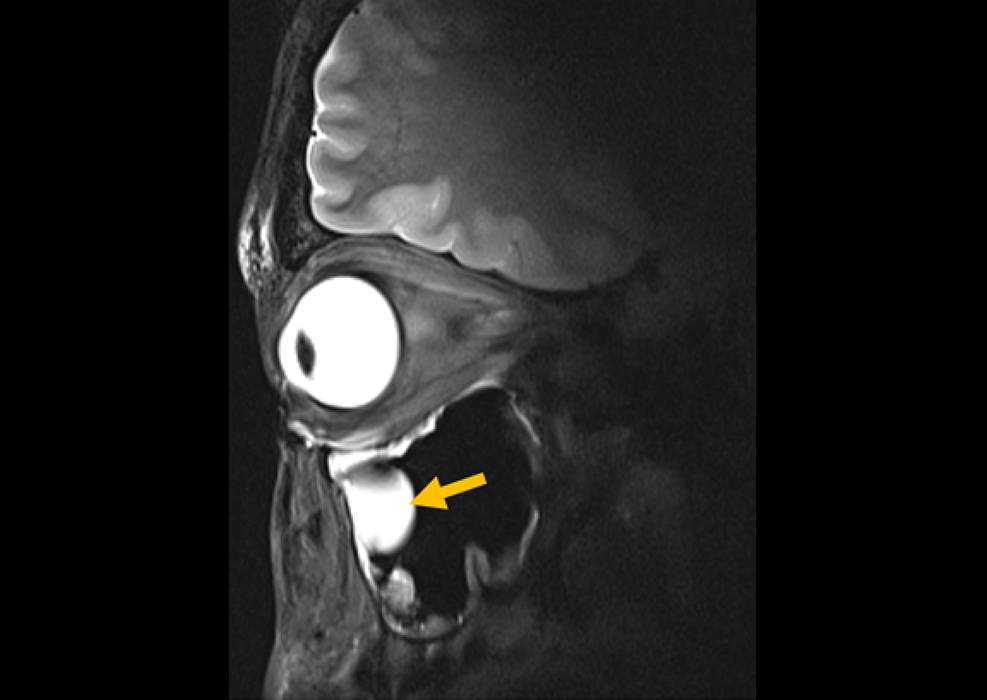
Contrast-enhanced magnetic resonance imaging (MRI) utilizes contrast agents to illuminate specific tissues or abnormalities, leading to improved visualization and more comprehensive information. Integrating artificial intelligence (AI) into this process could enhance image interpretation accuracy and efficiency, bolster diagnostic capabilities, and optimize patient care. Now, a groundbreaking AI-powered software has been designed to significantly improve brain MRI, particularly in detecting small and poorly enhanced lesions.
AiMIFY, a collaborative software from Bracco Diagnostics (Milan, Italy) and Subtle Medical (Menlo Park, CA, USA), employs advanced AI technology to increase the contrast enhancement of brain MR images by up to two times the level achieved with a standard dose of gadolinium-based contrast agents (GBCAs). This enhancement allows radiologists and neuroradiologists to obtain clearer, more detailed images, enhancing the visibility of both small and large lesions compared to standard post-contrast images. The software's efficacy has been validated across a wide range of test data, encompassing various patient demographics, pathologies, lesion sizes, MRI scanner vendors, sequences, and acquisition orientations. AiMIFY has received FDA clearance as a Class II software as a medical device (SaMD) specifically for brain MRI.
"This FDA clearance marks a significant milestone for our innovative product, showcasing its potential to transform MRI," said Fulvio Renoldi Bracco, Vice Chairman & Chief Executive Officer of Bracco Imaging S.p.A. "By integrating Bracco's expertise in contrast imaging with Subtle Medical's cutting-edge deep-learning technology, we are poised to redefine diagnostic precision and efficiency, setting new standards in the field for the ultimate benefit of the patients."
"We partnered with Bracco to unlock the potential that AI brings to medical imaging," added Ajit Shankaranarayanan, PhD, Chief Product Officer at Subtle Medical. "The FDA clearance represents a significant milestone for both companies, as we join forces to empower radiological professionals and improve outcomes for patients worldwide with this innovative AI-powered medical imaging solution."
Related Links:
Bracco Diagnostics
Subtle Medical