New 3D Printing Technologies Improve Pediatric Osteotomy Planning
|
By MedImaging International staff writers Posted on 22 Mar 2017 |

Image: Two 3D-printed patient-specific radius and ulna osteotomy guides for surgery planning in children (Photo courtesy of Materialise).
The US Food and Drug Administration (FDA) approval of new 3D-printed patient-specific radius and ulna osteotomy guides for children aged seven years and older will help orthopedic surgeons plan and carry out complex surgery.
The 3D-printed guides are intended for use with 3D pre-operative planning to help surgeons improve the planning processes before pediatric surgery and help them during the surgery. Until now surgeons have used 2D X-Ray imaging for pediatric osteotomy planning. The 3D-printed guides are intended to benefit children with bone injuries, and those with natural deformities.
The 3D-printed guides were developed by Materialise, located in Leuven, Belgium. Materialise has more than 26 years of 3D printing experience, and offers a range of end-to-end 3D printing solutions for the orthopedic industry. Each 3D-printed surgical guide is patient-specific. Materialise also works with hospitals and surgeons around the world to develop and improve 3D-printed osteotomy guides for complex bone corrections in adults. With this US FDA approval Materialise can now also provide 3D-printed guides for use with pediatric surgery.
VP and GM of Materialise North America, Bryan Crutchfield, said, “In bringing this 3D printing technology to pediatric surgery, surgeons will have access to our clinical engineers’ wealth of experience developing osteotomy guides, helping them perform even the most complex bone corrections that will have a positive impact on the rest of the child’s life.”
The 3D-printed guides are intended for use with 3D pre-operative planning to help surgeons improve the planning processes before pediatric surgery and help them during the surgery. Until now surgeons have used 2D X-Ray imaging for pediatric osteotomy planning. The 3D-printed guides are intended to benefit children with bone injuries, and those with natural deformities.
The 3D-printed guides were developed by Materialise, located in Leuven, Belgium. Materialise has more than 26 years of 3D printing experience, and offers a range of end-to-end 3D printing solutions for the orthopedic industry. Each 3D-printed surgical guide is patient-specific. Materialise also works with hospitals and surgeons around the world to develop and improve 3D-printed osteotomy guides for complex bone corrections in adults. With this US FDA approval Materialise can now also provide 3D-printed guides for use with pediatric surgery.
VP and GM of Materialise North America, Bryan Crutchfield, said, “In bringing this 3D printing technology to pediatric surgery, surgeons will have access to our clinical engineers’ wealth of experience developing osteotomy guides, helping them perform even the most complex bone corrections that will have a positive impact on the rest of the child’s life.”
Latest Imaging IT News
- New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
- Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
- AI-Based Mammography Triage Software Helps Dramatically Improve Interpretation Process
- Artificial Intelligence (AI) Program Accurately Predicts Lung Cancer Risk from CT Images
- Image Management Platform Streamlines Treatment Plans
- AI-Based Technology for Ultrasound Image Analysis Receives FDA Approval
- AI Technology for Detecting Breast Cancer Receives CE Mark Approval
- Digital Pathology Software Improves Workflow Efficiency
- Patient-Centric Portal Facilitates Direct Imaging Access
- New Workstation Supports Customer-Driven Imaging Workflow
Channels
Radiography
view channel
World's Largest Class Single Crystal Diamond Radiation Detector Opens New Possibilities for Diagnostic Imaging
Diamonds possess ideal physical properties for radiation detection, such as exceptional thermal and chemical stability along with a quick response time. Made of carbon with an atomic number of six, diamonds... Read more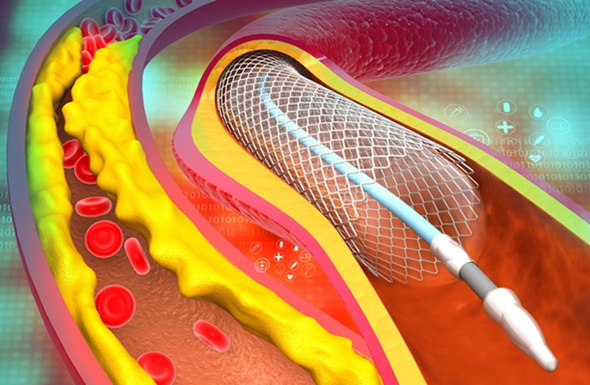
AI-Powered Imaging Technique Shows Promise in Evaluating Patients for PCI
Percutaneous coronary intervention (PCI), also known as coronary angioplasty, is a minimally invasive procedure where small metal tubes called stents are inserted into partially blocked coronary arteries... Read moreMRI
view channel
AI Tool Tracks Effectiveness of Multiple Sclerosis Treatments Using Brain MRI Scans
Multiple sclerosis (MS) is a condition in which the immune system attacks the brain and spinal cord, leading to impairments in movement, sensation, and cognition. Magnetic Resonance Imaging (MRI) markers... Read more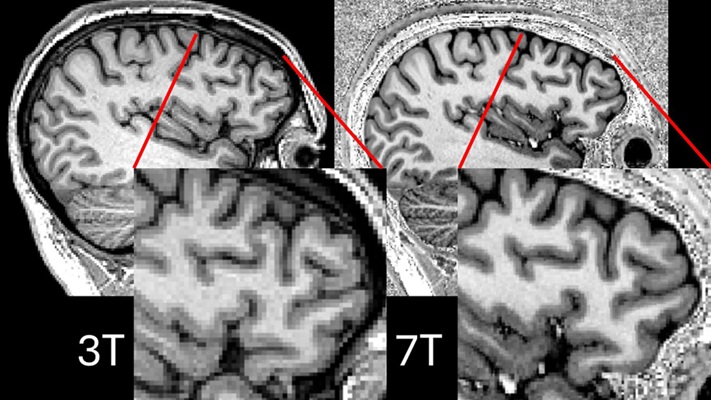
Ultra-Powerful MRI Scans Enable Life-Changing Surgery in Treatment-Resistant Epileptic Patients
Approximately 360,000 individuals in the UK suffer from focal epilepsy, a condition in which seizures spread from one part of the brain. Around a third of these patients experience persistent seizures... Read more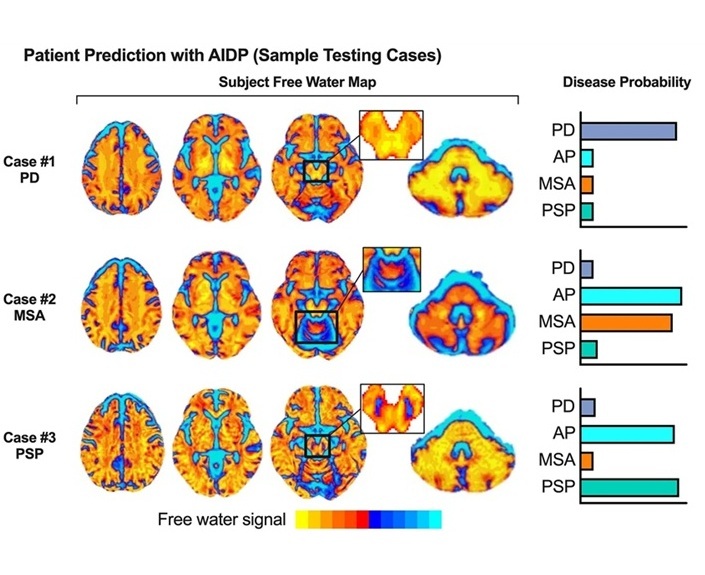
AI-Powered MRI Technology Improves Parkinson’s Diagnoses
Current research shows that the accuracy of diagnosing Parkinson’s disease typically ranges from 55% to 78% within the first five years of assessment. This is partly due to the similarities shared by Parkinson’s... Read more
Biparametric MRI Combined with AI Enhances Detection of Clinically Significant Prostate Cancer
Artificial intelligence (AI) technologies are transforming the way medical images are analyzed, offering unprecedented capabilities in quantitatively extracting features that go beyond traditional visual... Read moreUltrasound
view channel
AI Identifies Heart Valve Disease from Common Imaging Test
Tricuspid regurgitation is a condition where the heart's tricuspid valve does not close completely during contraction, leading to backward blood flow, which can result in heart failure. A new artificial... Read more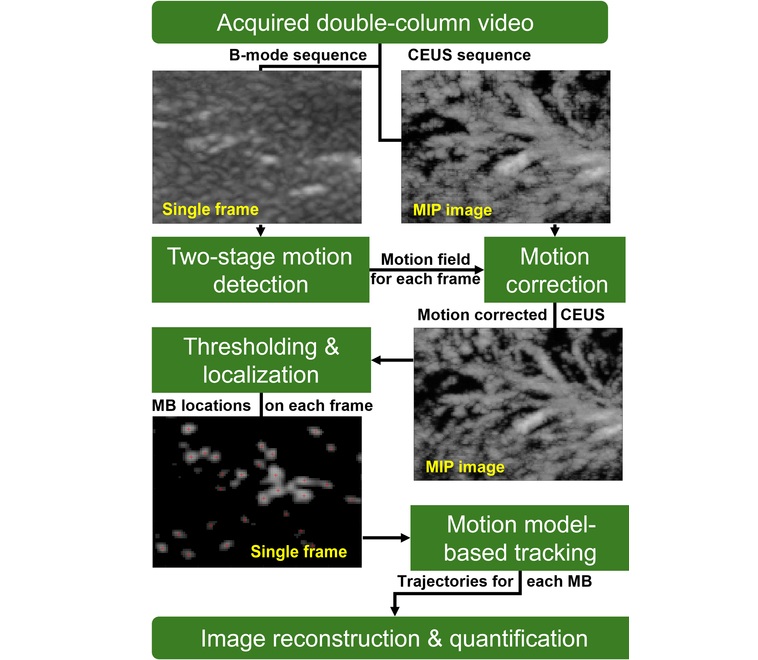
Novel Imaging Method Enables Early Diagnosis and Treatment Monitoring of Type 2 Diabetes
Type 2 diabetes is recognized as an autoimmune inflammatory disease, where chronic inflammation leads to alterations in pancreatic islet microvasculature, a key factor in β-cell dysfunction.... Read moreNuclear Medicine
view channel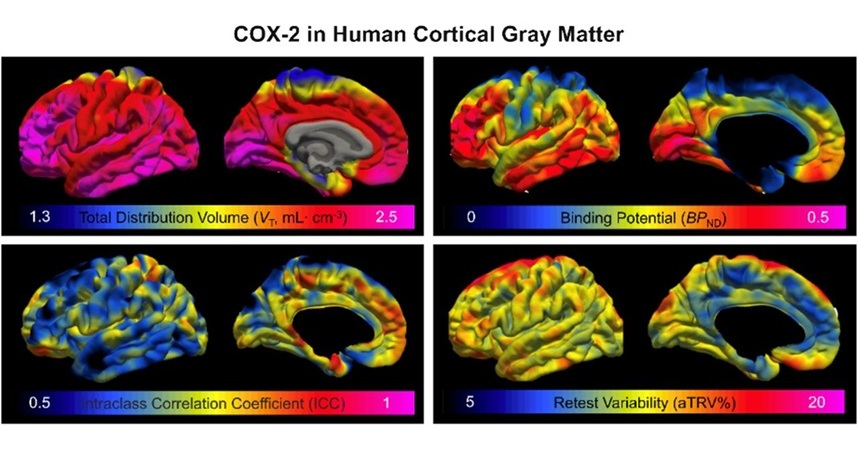
Novel PET Imaging Approach Offers Never-Before-Seen View of Neuroinflammation
COX-2, an enzyme that plays a key role in brain inflammation, can be significantly upregulated by inflammatory stimuli and neuroexcitation. Researchers suggest that COX-2 density in the brain could serve... Read more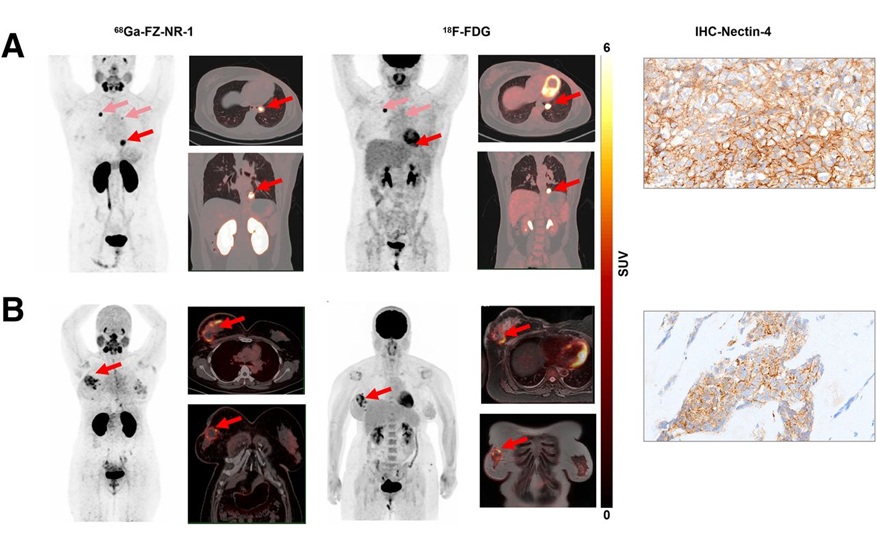
Novel Radiotracer Identifies Biomarker for Triple-Negative Breast Cancer
Triple-negative breast cancer (TNBC), which represents 15-20% of all breast cancer cases, is one of the most aggressive subtypes, with a five-year survival rate of about 40%. Due to its significant heterogeneity... Read moreGeneral/Advanced Imaging
view channel
AI-Powered Imaging System Improves Lung Cancer Diagnosis
Given the need to detect lung cancer at earlier stages, there is an increasing need for a definitive diagnostic pathway for patients with suspicious pulmonary nodules. However, obtaining tissue samples... Read more
AI Model Significantly Enhances Low-Dose CT Capabilities
Lung cancer remains one of the most challenging diseases, making early diagnosis vital for effective treatment. Fortunately, advancements in artificial intelligence (AI) are revolutionizing lung cancer... Read moreIndustry News
view channel
GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
GE HealthCare (Chicago, IL, USA) has entered into a collaboration with NVIDIA (Santa Clara, CA, USA), expanding the existing relationship between the two companies to focus on pioneering innovation in... Read more
Patient-Specific 3D-Printed Phantoms Transform CT Imaging
New research has highlighted how anatomically precise, patient-specific 3D-printed phantoms are proving to be scalable, cost-effective, and efficient tools in the development of new CT scan algorithms... Read more
Siemens and Sectra Collaborate on Enhancing Radiology Workflows
Siemens Healthineers (Forchheim, Germany) and Sectra (Linköping, Sweden) have entered into a collaboration aimed at enhancing radiologists' diagnostic capabilities and, in turn, improving patient care... Read more