NMR Innovation Offers Insights into Protein Interaction
|
By MedImaging International staff writers Posted on 17 Jul 2013 |
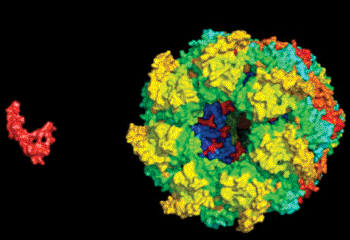
Image: A permissive captor A computer rendering depicts a GroEL protein hunting down an Aβ protein. Four complementary styles of nuclear magnetic resonance spectroscopy contributed to an understanding of the protein-to-protein interaction (Photo courtesy of Fawzi lab at Brown University).
By taking a novel approach to nuclear magnetic resonance spectroscopy--a fusion of four techniques--scientists have been able to resolve a key interaction between two proteins that could never be seen before.
The findings were published the week of June 24, 2013, in the Proceedings of the National Academy of Sciences of the United States of America (PNAS). The interaction the researchers became the first ones to describe is nearly universal across all of life. A protein unit called a chaperone takes hold of a disordered smaller protein to help it find its correct folded conformation. The scientists, in this instance, initiated test-tube experiments where they hoped to visualize the capsule-shaped bacterial chaperone GroEL capture a disordered amyloid beta (A-beta) protein, a molecule that in humans is central in Alzheimer's disease.
The two proteins are well researched, but the motions they go through when they first meet, i.e., when the open GroEL capsule captures its target, have been invisible to scientists. Electron microscopy and X-ray crystallography are only good for taking snapshots of easily frozen moments in time. NMR is capable of sensing the interactions and kinetics of protein interactions as they occur, but in some cases, any single technique can provide only clues into what is actually going on.
Brown University (Providence, RI, USA) biologist Dr. Nicolas Fawzi, who was a post-doc in the group of Marius Clore’s at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) within the US National Institutes of Health (NIH; Bethesda, MD, USA), worked with coauthors and NIDDK researchers Drs. David Libich, Jinfa Yang, and Marius Clore assembling the interactions of the proteins by combining four different NMR techniques. They determined what each one could inform them about the interaction and built the case presented in PNAS.
“None of the four techniques alone gave us sufficient information,” said Dr. Fawzi, now an assistant professor in Brown’s department of molecular pharmacology, physiology, and biotechnology. “Only by using them all together would we be able to figure out the structure and motions of A-beta; when it was bound to GroEL. By having four indirect measurements together, that was able to give us a complete picture.”
The NMR techniques they used were lifetime line broadening, Carr-Purcell-Meinboom-Gill (CPMG) relaxation dispersion spectroscopy, and exchange-induced chemical shifts. “The fourth technique we employed was dark-state exchange saturation transfer [DEST] spectroscopy, which we had developed in my lab at the NIH in 2011,” said Dr. Clore, also the article’s corresponding author. “We were able to more effectively conduct our research by using that tool to corroborate and extend the information afforded by the other three measurements.”
The elusive process debated among molecular biologists was about what the GroEL chaperone requires of its captives at the moment they engage. Does it force them into a specific conformation? Does it hold on tightly while it closes its capsule lid around the smaller protein, or does the captive stay in motion at all?
What the investigators observed is that the GroEL is a permissive captor. It bound A-beta; at just two hydrophobic sites, leaving the smaller protein to otherwise swing in a range of conformations. It also did not keep it bound the entire time, letting it instead detach and re-bind. Basically, A-beta would jump off and on within GroEL’s binding cavity.
“By using these four techniques together we were able to extract information about the structure of the protein while it binds as well as how fast it comes on and off and what it's doing at each position,” Dr. Fawzi said. “Instead of forming more particular structure upon binding it appears to retain great conformational heterogeneity.”
The lifetime line-broadening technique, for example, informed them that the A-beta; was interacting with something big (GroEL), while the CPMG and chemical shift observations combined to show the length of time A-beta; spent on GroEL before unbinding, as well as the structural characteristics of A-beta; when it was bound to GroEL. DEST provided data that could validate much of the story of the other techniques.
Dr. Fawzi noted that GroEL’s relaxed strategy could be a matter of being able to bind many different proteins in disordered conformations, but also of saving energy. Forcing proteins into a specific conformation just to make and sustain the initial capture would require more energy than it is worth. Eventually, in moments after those the team resolved in this study, GroEL closes its lid and encapsulates its target proteins fully, according to Dr. Fawzi said. Thatis when it capitalizes on in compelling them to fold in the correct manner.
For molecular and structural biologists, the newly proven combination of NMR techniques could create a number of other cold cases of elusive interactions. “We can now look at how these big machines can do their job while they are working,” Dr. Fawzi said. “This is not just limited to this GroEL machine.”
Related Links:
Brown University
The findings were published the week of June 24, 2013, in the Proceedings of the National Academy of Sciences of the United States of America (PNAS). The interaction the researchers became the first ones to describe is nearly universal across all of life. A protein unit called a chaperone takes hold of a disordered smaller protein to help it find its correct folded conformation. The scientists, in this instance, initiated test-tube experiments where they hoped to visualize the capsule-shaped bacterial chaperone GroEL capture a disordered amyloid beta (A-beta) protein, a molecule that in humans is central in Alzheimer's disease.
The two proteins are well researched, but the motions they go through when they first meet, i.e., when the open GroEL capsule captures its target, have been invisible to scientists. Electron microscopy and X-ray crystallography are only good for taking snapshots of easily frozen moments in time. NMR is capable of sensing the interactions and kinetics of protein interactions as they occur, but in some cases, any single technique can provide only clues into what is actually going on.
Brown University (Providence, RI, USA) biologist Dr. Nicolas Fawzi, who was a post-doc in the group of Marius Clore’s at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) within the US National Institutes of Health (NIH; Bethesda, MD, USA), worked with coauthors and NIDDK researchers Drs. David Libich, Jinfa Yang, and Marius Clore assembling the interactions of the proteins by combining four different NMR techniques. They determined what each one could inform them about the interaction and built the case presented in PNAS.
“None of the four techniques alone gave us sufficient information,” said Dr. Fawzi, now an assistant professor in Brown’s department of molecular pharmacology, physiology, and biotechnology. “Only by using them all together would we be able to figure out the structure and motions of A-beta; when it was bound to GroEL. By having four indirect measurements together, that was able to give us a complete picture.”
The NMR techniques they used were lifetime line broadening, Carr-Purcell-Meinboom-Gill (CPMG) relaxation dispersion spectroscopy, and exchange-induced chemical shifts. “The fourth technique we employed was dark-state exchange saturation transfer [DEST] spectroscopy, which we had developed in my lab at the NIH in 2011,” said Dr. Clore, also the article’s corresponding author. “We were able to more effectively conduct our research by using that tool to corroborate and extend the information afforded by the other three measurements.”
The elusive process debated among molecular biologists was about what the GroEL chaperone requires of its captives at the moment they engage. Does it force them into a specific conformation? Does it hold on tightly while it closes its capsule lid around the smaller protein, or does the captive stay in motion at all?
What the investigators observed is that the GroEL is a permissive captor. It bound A-beta; at just two hydrophobic sites, leaving the smaller protein to otherwise swing in a range of conformations. It also did not keep it bound the entire time, letting it instead detach and re-bind. Basically, A-beta would jump off and on within GroEL’s binding cavity.
“By using these four techniques together we were able to extract information about the structure of the protein while it binds as well as how fast it comes on and off and what it's doing at each position,” Dr. Fawzi said. “Instead of forming more particular structure upon binding it appears to retain great conformational heterogeneity.”
The lifetime line-broadening technique, for example, informed them that the A-beta; was interacting with something big (GroEL), while the CPMG and chemical shift observations combined to show the length of time A-beta; spent on GroEL before unbinding, as well as the structural characteristics of A-beta; when it was bound to GroEL. DEST provided data that could validate much of the story of the other techniques.
Dr. Fawzi noted that GroEL’s relaxed strategy could be a matter of being able to bind many different proteins in disordered conformations, but also of saving energy. Forcing proteins into a specific conformation just to make and sustain the initial capture would require more energy than it is worth. Eventually, in moments after those the team resolved in this study, GroEL closes its lid and encapsulates its target proteins fully, according to Dr. Fawzi said. Thatis when it capitalizes on in compelling them to fold in the correct manner.
For molecular and structural biologists, the newly proven combination of NMR techniques could create a number of other cold cases of elusive interactions. “We can now look at how these big machines can do their job while they are working,” Dr. Fawzi said. “This is not just limited to this GroEL machine.”
Related Links:
Brown University
Latest Nuclear Medicine News
- Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
- Novel PET Imaging Approach Offers Never-Before-Seen View of Neuroinflammation
- Novel Radiotracer Identifies Biomarker for Triple-Negative Breast Cancer
- Innovative PET Imaging Technique to Help Diagnose Neurodegeneration
- New Molecular Imaging Test to Improve Lung Cancer Diagnosis
- Novel PET Technique Visualizes Spinal Cord Injuries to Predict Recovery
- Next-Gen Tau Radiotracers Outperform FDA-Approved Imaging Agents in Detecting Alzheimer’s
- Breakthrough Method Detects Inflammation in Body Using PET Imaging
- Advanced Imaging Reveals Hidden Metastases in High-Risk Prostate Cancer Patients
- Combining Advanced Imaging Technologies Offers Breakthrough in Glioblastoma Treatment
- New Molecular Imaging Agent Accurately Identifies Crucial Cancer Biomarker
- New Scans Light Up Aggressive Tumors for Better Treatment
- AI Stroke Brain Scan Readings Twice as Accurate as Current Method
- AI Analysis of PET/CT Images Predicts Side Effects of Immunotherapy in Lung Cancer
- New Imaging Agent to Drive Step-Change for Brain Cancer Imaging
- Portable PET Scanner to Detect Earliest Stages of Alzheimer’s Disease
Channels
Radiography
view channel
AI Improves Early Detection of Interval Breast Cancers
Interval breast cancers, which occur between routine screenings, are easier to treat when detected earlier. Early detection can reduce the need for aggressive treatments and improve the chances of better outcomes.... Read more
World's Largest Class Single Crystal Diamond Radiation Detector Opens New Possibilities for Diagnostic Imaging
Diamonds possess ideal physical properties for radiation detection, such as exceptional thermal and chemical stability along with a quick response time. Made of carbon with an atomic number of six, diamonds... Read moreMRI
view channel
Cutting-Edge MRI Technology to Revolutionize Diagnosis of Common Heart Problem
Aortic stenosis is a common and potentially life-threatening heart condition. It occurs when the aortic valve, which regulates blood flow from the heart to the rest of the body, becomes stiff and narrow.... Read more
New MRI Technique Reveals True Heart Age to Prevent Attacks and Strokes
Heart disease remains one of the leading causes of death worldwide. Individuals with conditions such as diabetes or obesity often experience accelerated aging of their hearts, sometimes by decades.... Read more
AI Tool Predicts Relapse of Pediatric Brain Cancer from Brain MRI Scans
Many pediatric gliomas are treatable with surgery alone, but relapses can be catastrophic. Predicting which patients are at risk for recurrence remains challenging, leading to frequent follow-ups with... Read more
AI Tool Tracks Effectiveness of Multiple Sclerosis Treatments Using Brain MRI Scans
Multiple sclerosis (MS) is a condition in which the immune system attacks the brain and spinal cord, leading to impairments in movement, sensation, and cognition. Magnetic Resonance Imaging (MRI) markers... Read moreUltrasound
view channel.jpeg)
AI-Powered Lung Ultrasound Outperforms Human Experts in Tuberculosis Diagnosis
Despite global declines in tuberculosis (TB) rates in previous years, the incidence of TB rose by 4.6% from 2020 to 2023. Early screening and rapid diagnosis are essential elements of the World Health... Read more
AI Identifies Heart Valve Disease from Common Imaging Test
Tricuspid regurgitation is a condition where the heart's tricuspid valve does not close completely during contraction, leading to backward blood flow, which can result in heart failure. A new artificial... Read moreNuclear Medicine
view channel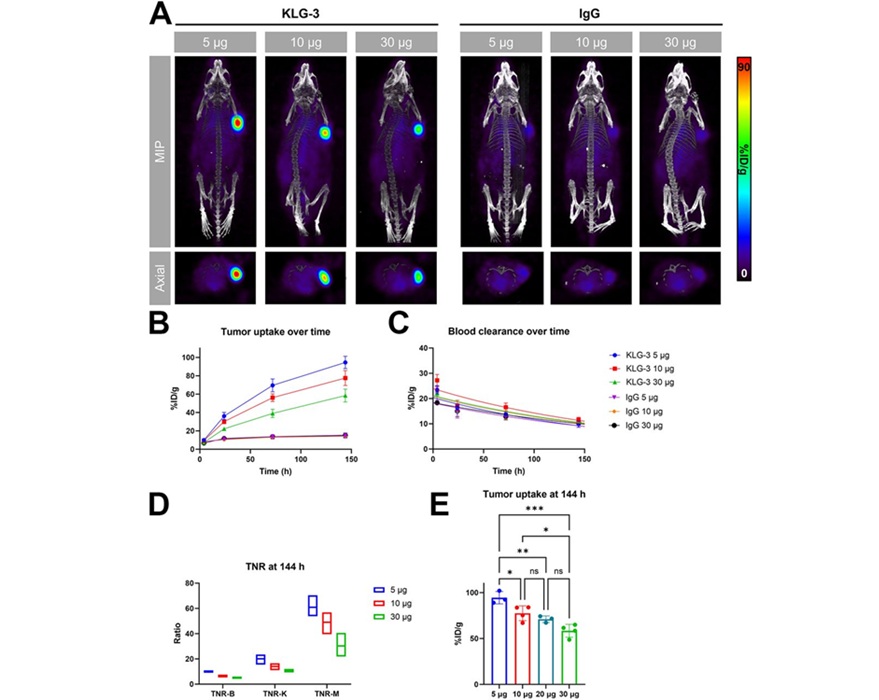
Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
Interleukin-13 receptor α-2 (IL13Rα2) is a cell surface receptor commonly found in solid tumors such as glioblastoma, melanoma, and breast cancer. It is minimally expressed in normal tissues, making it... Read more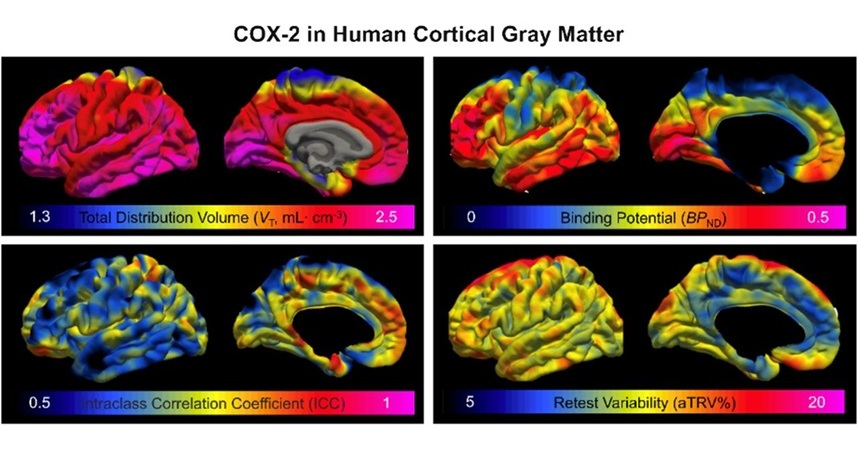
Novel PET Imaging Approach Offers Never-Before-Seen View of Neuroinflammation
COX-2, an enzyme that plays a key role in brain inflammation, can be significantly upregulated by inflammatory stimuli and neuroexcitation. Researchers suggest that COX-2 density in the brain could serve... Read moreGeneral/Advanced Imaging
view channel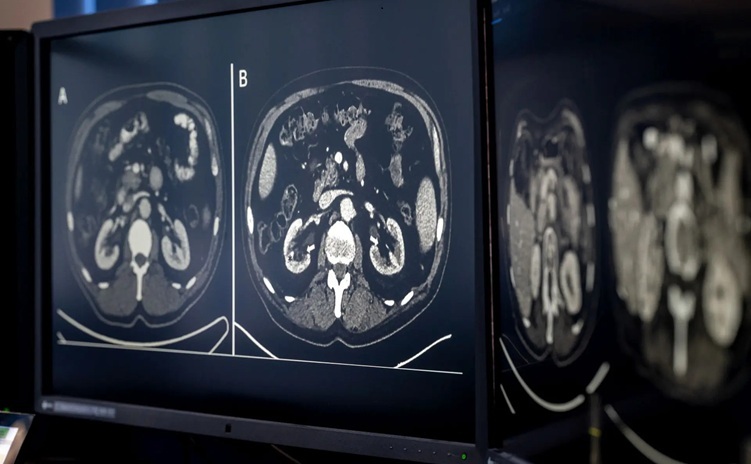
AI-Based CT Scan Analysis Predicts Early-Stage Kidney Damage Due to Cancer Treatments
Radioligand therapy, a form of targeted nuclear medicine, has recently gained attention for its potential in treating specific types of tumors. However, one of the potential side effects of this therapy... Read more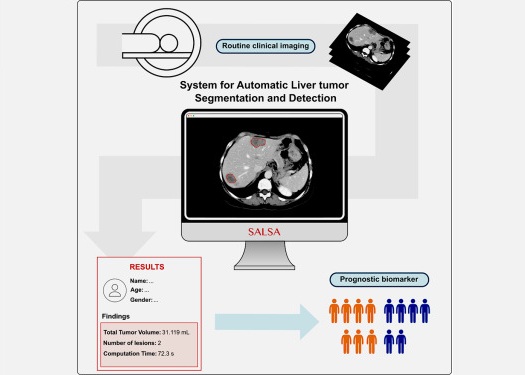
CT-Based Deep Learning-Driven Tool to Enhance Liver Cancer Diagnosis
Medical imaging, such as computed tomography (CT) scans, plays a crucial role in oncology, offering essential data for cancer detection, treatment planning, and monitoring of response to therapies.... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel
GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
GE HealthCare (Chicago, IL, USA) has entered into a collaboration with NVIDIA (Santa Clara, CA, USA), expanding the existing relationship between the two companies to focus on pioneering innovation in... Read more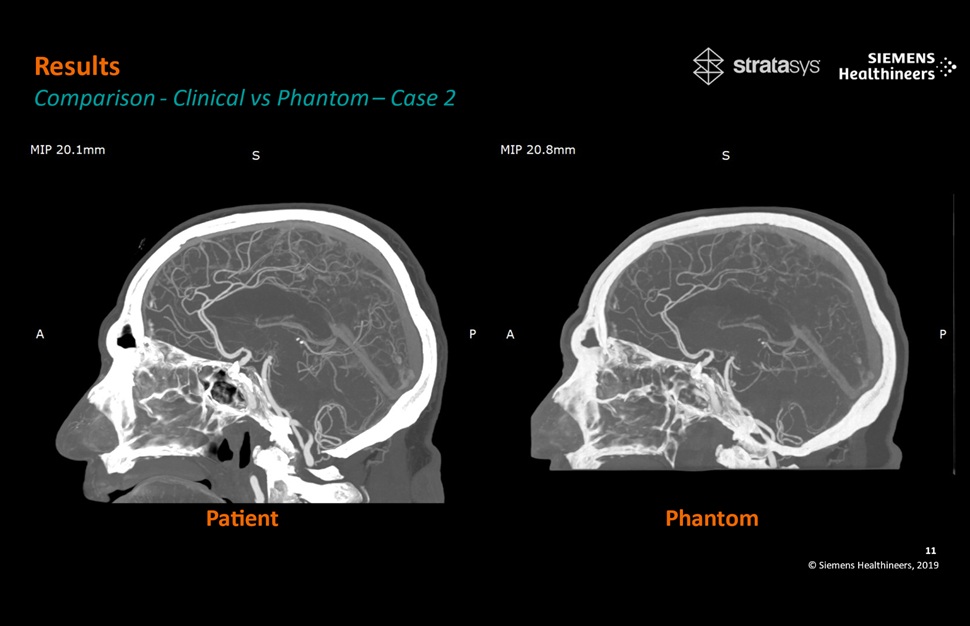
Patient-Specific 3D-Printed Phantoms Transform CT Imaging
New research has highlighted how anatomically precise, patient-specific 3D-printed phantoms are proving to be scalable, cost-effective, and efficient tools in the development of new CT scan algorithms... Read more
Siemens and Sectra Collaborate on Enhancing Radiology Workflows
Siemens Healthineers (Forchheim, Germany) and Sectra (Linköping, Sweden) have entered into a collaboration aimed at enhancing radiologists' diagnostic capabilities and, in turn, improving patient care... Read more




 Guided Devices.jpg)