New Imaging Agent to Drive Step-Change for Brain Cancer Imaging
Posted on 07 Nov 2024
Gliomas are highly diffusely infiltrative tumors that impact the surrounding brain tissue. They represent the most prevalent type of central nervous system (CNS) neoplasm originating from glial cells, accounting for around 30% of all brain and CNS tumors and approximately 80% of malignant brain tumors. There is a significant unmet need to enhance the diagnosis and management of gliomas, particularly after treatment. Traditional MRI imaging techniques have various limitations, such as a lack of biological specificity, reliance on the disruption of the blood-brain barrier, and an intrinsic inability to distinguish between tumor progression and treatment-related changes. These issues can lead to inconclusive results, delaying critical treatment decisions. With low survival rates and the necessity for prompt actions, precision imaging becomes essential. Now, a new PET imaging agent for gliomas has the potential to meet this need, providing patients with clearer diagnoses and aiding treatment decision-making.
Telix Pharmaceuticals (Melbourne, Australia) has developed Pixclara (18F-floretyrosine or 18F-FET), a PET agent designed to differentiate between progressive or recurrent gliomas and treatment-related changes in both adult and pediatric patients. The U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA) for TLX101-CDx (Pixclara), an imaging agent for gliomas. This application has been given priority review status, with a Prescription Drug User Fee Act (PDUFA) goal date set for April 26, 2025, facilitating a potential U.S. commercial launch in 2025.
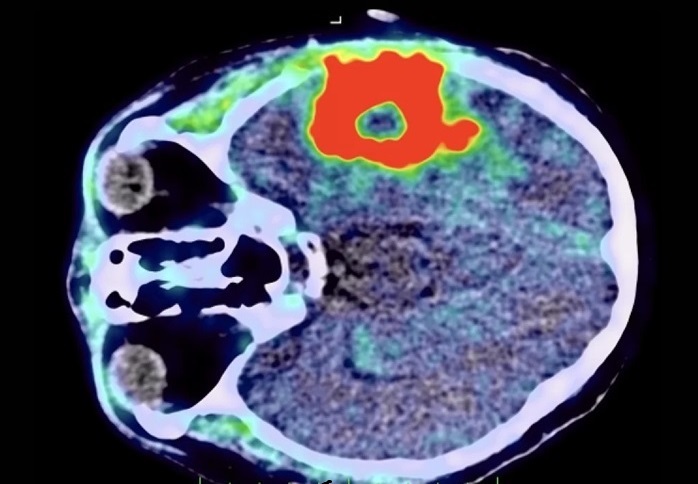
While FET PET is already part of international clinical practice guidelines for glioma imaging, there is currently no FDA-approved targeted amino acid PET agent available for adult and pediatric brain cancer imaging in the U.S. Given its potential to fulfill a significant medical need, Pixclara has been designated as an orphan drug and granted fast track designation by the FDA. Telix is also exploring the possibility of using Pixclara as a companion diagnostic agent for TLX101-Tx, an investigational neuro-oncology drug in development that targets the same amino acid transporter mechanism with therapeutic radiation.
“Telix believes that the FDA approval of Pixclara will drive a step-change for brain cancer imaging in the U.S., and bring it into line with a more advanced standard of care currently used in other markets,” said Kevin Richardson, Chief Executive Officer, Telix Precision Medicine. “There is currently a critical need for better imaging in brain cancer, and Telix is dedicated to delivering precision medicine solutions that address patient needs and enhance both cancer imaging and treatment outcomes.”
Related Links:
Telix Pharmaceuticals