Further Steps Taken to Provide Clear Guidelines for PET Radiopharmaceuticals
|
By MedImaging International staff writers Posted on 20 Jan 2010 |
U.S. officials have announced publication of a final regulation on current good manufacturing practices (cGMPs) for the production of positron emission tomography (PET) imaging agents, as well as a guidance document describing acceptable approaches that would enable PET drug manufacturers to meet the requirements in the proposed regulation.
"We are pleased that FDA [U.S. Food and Drug Administration (Silver Springs, MD, USA)] has issued cGMPs for PET drugs, which are so important to the diagnosis and treatment of patients with heart disease, cancer, and other life-threatening illnesses,” said Michael M. Graham, Ph.D., M.D., president of SNM (Society of Nuclear Medicine; Reston, VA, USA) and director of nuclear medicine at the University of Iowa Carver College of Medicine (Iowa City, USA).
The new FDA guidelines, which took effect December 11, 2011, are aimed at ensuring that PET drugs meet all requirements of safety, identity, strength, quality, and purity. The cGMP guidance document describes acceptable approaches that would enable PET drug producers to meet regulatory requirements. All PET drug manufacturers will be required to submit a new drug application (NDA) or abbreviated new drug application (aNDA) for all PET drug products in routine clinical use by the date of implementation. In the interim, U.S. facilities must continue to comply with U.S. Phamacopeia <823>, which sets standards for the production of PET drugs.
SNM worked in concert with other medical organizations to provide FDA input and review on the cGMP guidelines. "This is a major step forward,” said Dr. Graham. "Having a well-defined structure in place benefits manufacturers, physicians, and patients by ensuring the highest quality drugs possible.”
Through its Clinical Trials Network, SNM will offer educational programs on the new regulation. Representatives from FDA will discuss the new guidelines with the molecular imaging and manufacturing community at two upcoming events. FDA representatives will present a special session during the Clinical Trials Network Workshop, which will be held Feb. 1, 2010, at SNM's Conjoint Mid-Winter Meetings in Albuquerque, NM, USA. In addition, FDA representatives will present a half-day workshop at SNM's annual meeting, June 5, 2010, in Salt Lake City, UT, USA. To date, more than 130 manufacturing sites have registered with the Clinical Trials Network.
Related Links:
U.S. Food and Drug Administration
SNM
"We are pleased that FDA [U.S. Food and Drug Administration (Silver Springs, MD, USA)] has issued cGMPs for PET drugs, which are so important to the diagnosis and treatment of patients with heart disease, cancer, and other life-threatening illnesses,” said Michael M. Graham, Ph.D., M.D., president of SNM (Society of Nuclear Medicine; Reston, VA, USA) and director of nuclear medicine at the University of Iowa Carver College of Medicine (Iowa City, USA).
The new FDA guidelines, which took effect December 11, 2011, are aimed at ensuring that PET drugs meet all requirements of safety, identity, strength, quality, and purity. The cGMP guidance document describes acceptable approaches that would enable PET drug producers to meet regulatory requirements. All PET drug manufacturers will be required to submit a new drug application (NDA) or abbreviated new drug application (aNDA) for all PET drug products in routine clinical use by the date of implementation. In the interim, U.S. facilities must continue to comply with U.S. Phamacopeia <823>, which sets standards for the production of PET drugs.
SNM worked in concert with other medical organizations to provide FDA input and review on the cGMP guidelines. "This is a major step forward,” said Dr. Graham. "Having a well-defined structure in place benefits manufacturers, physicians, and patients by ensuring the highest quality drugs possible.”
Through its Clinical Trials Network, SNM will offer educational programs on the new regulation. Representatives from FDA will discuss the new guidelines with the molecular imaging and manufacturing community at two upcoming events. FDA representatives will present a special session during the Clinical Trials Network Workshop, which will be held Feb. 1, 2010, at SNM's Conjoint Mid-Winter Meetings in Albuquerque, NM, USA. In addition, FDA representatives will present a half-day workshop at SNM's annual meeting, June 5, 2010, in Salt Lake City, UT, USA. To date, more than 130 manufacturing sites have registered with the Clinical Trials Network.
Related Links:
U.S. Food and Drug Administration
SNM
Latest Nuclear Medicine News
- Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
- Novel PET Imaging Approach Offers Never-Before-Seen View of Neuroinflammation
- Novel Radiotracer Identifies Biomarker for Triple-Negative Breast Cancer
- Innovative PET Imaging Technique to Help Diagnose Neurodegeneration
- New Molecular Imaging Test to Improve Lung Cancer Diagnosis
- Novel PET Technique Visualizes Spinal Cord Injuries to Predict Recovery
- Next-Gen Tau Radiotracers Outperform FDA-Approved Imaging Agents in Detecting Alzheimer’s
- Breakthrough Method Detects Inflammation in Body Using PET Imaging
- Advanced Imaging Reveals Hidden Metastases in High-Risk Prostate Cancer Patients
- Combining Advanced Imaging Technologies Offers Breakthrough in Glioblastoma Treatment
- New Molecular Imaging Agent Accurately Identifies Crucial Cancer Biomarker
- New Scans Light Up Aggressive Tumors for Better Treatment
- AI Stroke Brain Scan Readings Twice as Accurate as Current Method
- AI Analysis of PET/CT Images Predicts Side Effects of Immunotherapy in Lung Cancer
- New Imaging Agent to Drive Step-Change for Brain Cancer Imaging
- Portable PET Scanner to Detect Earliest Stages of Alzheimer’s Disease
Channels
Radiography
view channel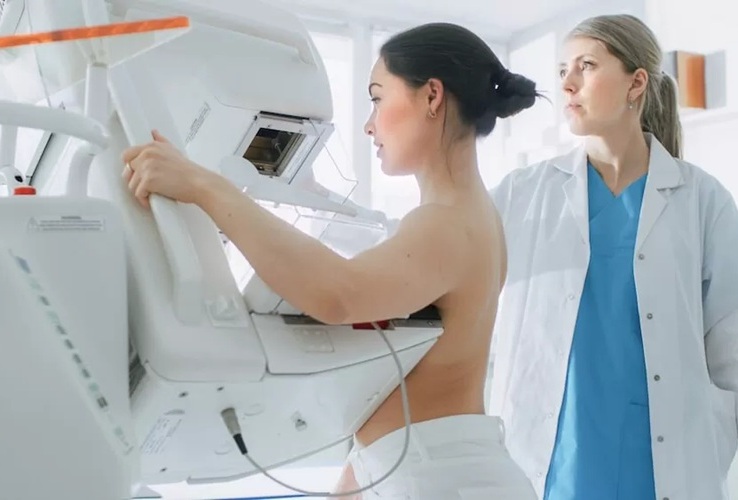
AI Improves Early Detection of Interval Breast Cancers
Interval breast cancers, which occur between routine screenings, are easier to treat when detected earlier. Early detection can reduce the need for aggressive treatments and improve the chances of better outcomes.... Read more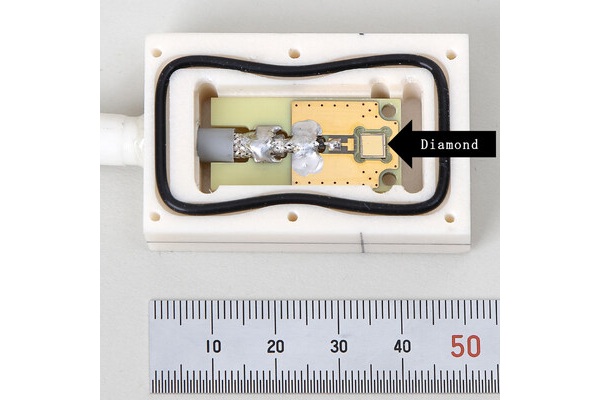
World's Largest Class Single Crystal Diamond Radiation Detector Opens New Possibilities for Diagnostic Imaging
Diamonds possess ideal physical properties for radiation detection, such as exceptional thermal and chemical stability along with a quick response time. Made of carbon with an atomic number of six, diamonds... Read moreMRI
view channel
Cutting-Edge MRI Technology to Revolutionize Diagnosis of Common Heart Problem
Aortic stenosis is a common and potentially life-threatening heart condition. It occurs when the aortic valve, which regulates blood flow from the heart to the rest of the body, becomes stiff and narrow.... Read more
New MRI Technique Reveals True Heart Age to Prevent Attacks and Strokes
Heart disease remains one of the leading causes of death worldwide. Individuals with conditions such as diabetes or obesity often experience accelerated aging of their hearts, sometimes by decades.... Read more
AI Tool Predicts Relapse of Pediatric Brain Cancer from Brain MRI Scans
Many pediatric gliomas are treatable with surgery alone, but relapses can be catastrophic. Predicting which patients are at risk for recurrence remains challenging, leading to frequent follow-ups with... Read more
AI Tool Tracks Effectiveness of Multiple Sclerosis Treatments Using Brain MRI Scans
Multiple sclerosis (MS) is a condition in which the immune system attacks the brain and spinal cord, leading to impairments in movement, sensation, and cognition. Magnetic Resonance Imaging (MRI) markers... Read moreUltrasound
view channel.jpeg)
AI-Powered Lung Ultrasound Outperforms Human Experts in Tuberculosis Diagnosis
Despite global declines in tuberculosis (TB) rates in previous years, the incidence of TB rose by 4.6% from 2020 to 2023. Early screening and rapid diagnosis are essential elements of the World Health... Read more
AI Identifies Heart Valve Disease from Common Imaging Test
Tricuspid regurgitation is a condition where the heart's tricuspid valve does not close completely during contraction, leading to backward blood flow, which can result in heart failure. A new artificial... Read moreGeneral/Advanced Imaging
view channel
AI-Based CT Scan Analysis Predicts Early-Stage Kidney Damage Due to Cancer Treatments
Radioligand therapy, a form of targeted nuclear medicine, has recently gained attention for its potential in treating specific types of tumors. However, one of the potential side effects of this therapy... Read more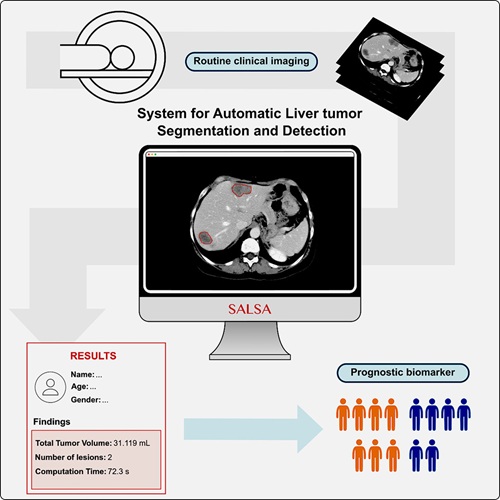
CT-Based Deep Learning-Driven Tool to Enhance Liver Cancer Diagnosis
Medical imaging, such as computed tomography (CT) scans, plays a crucial role in oncology, offering essential data for cancer detection, treatment planning, and monitoring of response to therapies.... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel
GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
GE HealthCare (Chicago, IL, USA) has entered into a collaboration with NVIDIA (Santa Clara, CA, USA), expanding the existing relationship between the two companies to focus on pioneering innovation in... Read more
Patient-Specific 3D-Printed Phantoms Transform CT Imaging
New research has highlighted how anatomically precise, patient-specific 3D-printed phantoms are proving to be scalable, cost-effective, and efficient tools in the development of new CT scan algorithms... Read more
Siemens and Sectra Collaborate on Enhancing Radiology Workflows
Siemens Healthineers (Forchheim, Germany) and Sectra (Linköping, Sweden) have entered into a collaboration aimed at enhancing radiologists' diagnostic capabilities and, in turn, improving patient care... Read more