Ethicists Discourage Use of "Unproven" Screening Tests
|
By MedImaging International staff writers Posted on 21 Apr 2009 |
Ethicists have concluded that whole-body computed tomography (CT) scans and other, what they deem unproven, screening tests have significant risks, and physicians should generally discourage their use when such tests lack data and professional support for their use.
Private companies offer access to these new tests--such as lung scans for smokers, magnetic resonance angiography (MRA) to detect cerebral aneurysm, and whole-body CT--without referrals from a primary care physician. But such testing creates ethical problems, according to the authors of the report, which was published in the March 31, 2009, issue of The American Journal of Bioethics. "Under most circumstances, physicians should discourage individual patient access to screening exams prior to conclusive evidence and professional society endorsement," stated Drs. Ingrid Burger and Nancy Kass, from Johns Hopkins University (Baltimore, MD, USA).
Good evidence supports the use of other screening tests such as colonoscopies, pap smears, and mammograms, according to Drs. Burger and Kass. But problems arise, they reported, when entrepreneurs and doctors encourage access to whole-body CT, which may detect cancer early but has never been proven to save lives. "CT exposes patients to radiation, and high false-positive rates may lead to invasive, risky, and costly follow-up procedures," said Drs. Burger and Kass. "Further, the detection of pseudo disease--that is, cancers that grow so slowly that they never produce symptoms or impact a patient's health---leads to interventions and treatments that provide no medical benefit and may pose significant risk."
Widespread adoption of unproven tests outside clinical trials may also slow research to characterize their risks and benefits, the investigators reported in their article. Some such trials are underway, such as the U.S. National Lung Screening Trial, which has enrolled 50,000 smokers to see whether CT screening for lung cancer can save lives. It will cost approximately US$200 million over eight years. "Randomized controlled trials of screening tests can require thousands of patients, years of follow-up, and high costs to complete," Drs. Burger and Kass conceded.
In light of these obstacles, the researchers contended that physicians may sometimes have compelling reasons to use an unproven screening test for high-risk patients who place a high value on the results. The authors proposed four guidelines to help doctors use new screening tests ethically before there is solid evidence to support their use: (1) Physicians should fully understand the epidemiologic basis for screening tests and the tendency of patients to overestimate the value of screening. (2) Physicians should understand the state of the evidence and professional recommendations surrounding screening tests. (3) Physicians should advocate for research to assess new screening tests and the tendency of patients to overestimate the value of screening. (4) Physicians should understand the state of the evidence and professional recommendations surrounding screening tests. (5) Physicians should advocate for research to evaluate new screening tests. (6) Physicians should not engage in direct-to-consumer advertising of screening tests.
"Consumers may falsely infer that any screening exam advertised by physicians offers more benefits than risks and/or is recommended by physicians as a whole," stated Drs. Burger and Kass. "Given the high likelihood of misleading patients, consumer advertising before screening is endorsed for a population, in our view, is unprofessional."
Related Links:
Johns Hopkins University
Private companies offer access to these new tests--such as lung scans for smokers, magnetic resonance angiography (MRA) to detect cerebral aneurysm, and whole-body CT--without referrals from a primary care physician. But such testing creates ethical problems, according to the authors of the report, which was published in the March 31, 2009, issue of The American Journal of Bioethics. "Under most circumstances, physicians should discourage individual patient access to screening exams prior to conclusive evidence and professional society endorsement," stated Drs. Ingrid Burger and Nancy Kass, from Johns Hopkins University (Baltimore, MD, USA).
Good evidence supports the use of other screening tests such as colonoscopies, pap smears, and mammograms, according to Drs. Burger and Kass. But problems arise, they reported, when entrepreneurs and doctors encourage access to whole-body CT, which may detect cancer early but has never been proven to save lives. "CT exposes patients to radiation, and high false-positive rates may lead to invasive, risky, and costly follow-up procedures," said Drs. Burger and Kass. "Further, the detection of pseudo disease--that is, cancers that grow so slowly that they never produce symptoms or impact a patient's health---leads to interventions and treatments that provide no medical benefit and may pose significant risk."
Widespread adoption of unproven tests outside clinical trials may also slow research to characterize their risks and benefits, the investigators reported in their article. Some such trials are underway, such as the U.S. National Lung Screening Trial, which has enrolled 50,000 smokers to see whether CT screening for lung cancer can save lives. It will cost approximately US$200 million over eight years. "Randomized controlled trials of screening tests can require thousands of patients, years of follow-up, and high costs to complete," Drs. Burger and Kass conceded.
In light of these obstacles, the researchers contended that physicians may sometimes have compelling reasons to use an unproven screening test for high-risk patients who place a high value on the results. The authors proposed four guidelines to help doctors use new screening tests ethically before there is solid evidence to support their use: (1) Physicians should fully understand the epidemiologic basis for screening tests and the tendency of patients to overestimate the value of screening. (2) Physicians should understand the state of the evidence and professional recommendations surrounding screening tests. (3) Physicians should advocate for research to assess new screening tests and the tendency of patients to overestimate the value of screening. (4) Physicians should understand the state of the evidence and professional recommendations surrounding screening tests. (5) Physicians should advocate for research to evaluate new screening tests. (6) Physicians should not engage in direct-to-consumer advertising of screening tests.
"Consumers may falsely infer that any screening exam advertised by physicians offers more benefits than risks and/or is recommended by physicians as a whole," stated Drs. Burger and Kass. "Given the high likelihood of misleading patients, consumer advertising before screening is endorsed for a population, in our view, is unprofessional."
Related Links:
Johns Hopkins University
Latest General/Advanced Imaging News
- AI-Powered Imaging System Improves Lung Cancer Diagnosis
- AI Model Significantly Enhances Low-Dose CT Capabilities
- Ultra-Low Dose CT Aids Pneumonia Diagnosis in Immunocompromised Patients
- AI Reduces CT Lung Cancer Screening Workload by Almost 80%
- Cutting-Edge Technology Combines Light and Sound for Real-Time Stroke Monitoring
- AI System Detects Subtle Changes in Series of Medical Images Over Time
- New CT Scan Technique to Improve Prognosis and Treatments for Head and Neck Cancers
- World’s First Mobile Whole-Body CT Scanner to Provide Diagnostics at POC
- Comprehensive CT Scans Could Identify Atherosclerosis Among Lung Cancer Patients
- AI Improves Detection of Colorectal Cancer on Routine Abdominopelvic CT Scans
- Super-Resolution Technology Enhances Clinical Bone Imaging to Predict Osteoporotic Fracture Risk
- AI-Powered Abdomen Map Enables Early Cancer Detection
- Deep Learning Model Detects Lung Tumors on CT
- AI Predicts Cardiovascular Risk from CT Scans
- Deep Learning Based Algorithms Improve Tumor Detection in PET/CT Scans
- New Technology Provides Coronary Artery Calcification Scoring on Ungated Chest CT Scans
Channels
Radiography
view channel
World's Largest Class Single Crystal Diamond Radiation Detector Opens New Possibilities for Diagnostic Imaging
Diamonds possess ideal physical properties for radiation detection, such as exceptional thermal and chemical stability along with a quick response time. Made of carbon with an atomic number of six, diamonds... Read more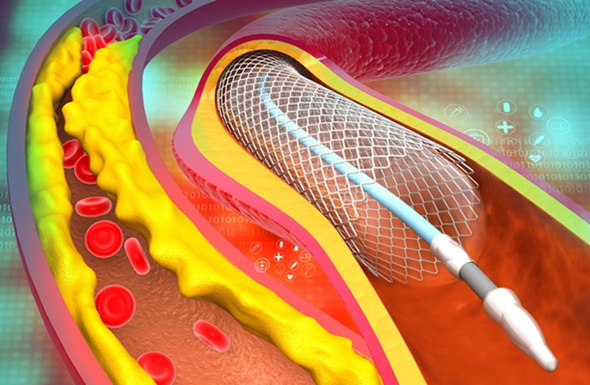
AI-Powered Imaging Technique Shows Promise in Evaluating Patients for PCI
Percutaneous coronary intervention (PCI), also known as coronary angioplasty, is a minimally invasive procedure where small metal tubes called stents are inserted into partially blocked coronary arteries... Read moreMRI
view channel
AI Tool Tracks Effectiveness of Multiple Sclerosis Treatments Using Brain MRI Scans
Multiple sclerosis (MS) is a condition in which the immune system attacks the brain and spinal cord, leading to impairments in movement, sensation, and cognition. Magnetic Resonance Imaging (MRI) markers... Read more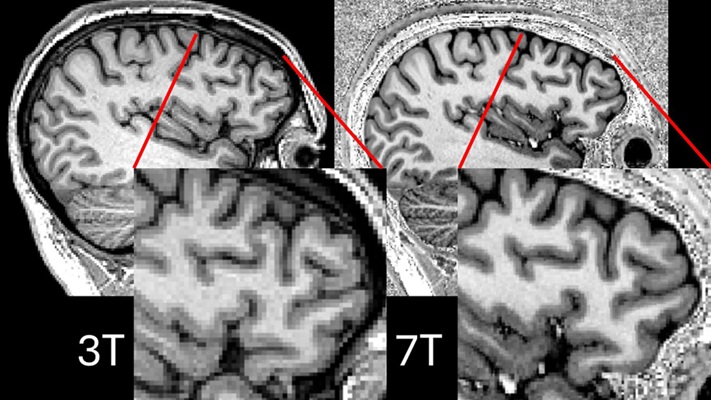
Ultra-Powerful MRI Scans Enable Life-Changing Surgery in Treatment-Resistant Epileptic Patients
Approximately 360,000 individuals in the UK suffer from focal epilepsy, a condition in which seizures spread from one part of the brain. Around a third of these patients experience persistent seizures... Read more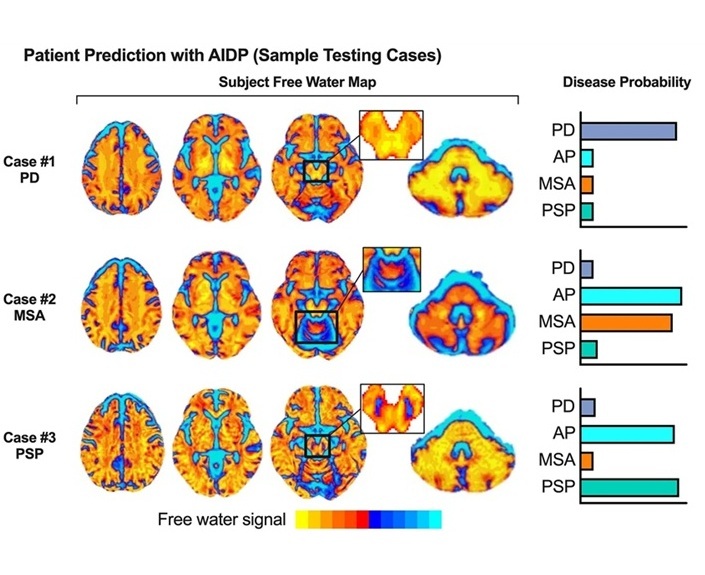
AI-Powered MRI Technology Improves Parkinson’s Diagnoses
Current research shows that the accuracy of diagnosing Parkinson’s disease typically ranges from 55% to 78% within the first five years of assessment. This is partly due to the similarities shared by Parkinson’s... Read more
Biparametric MRI Combined with AI Enhances Detection of Clinically Significant Prostate Cancer
Artificial intelligence (AI) technologies are transforming the way medical images are analyzed, offering unprecedented capabilities in quantitatively extracting features that go beyond traditional visual... Read moreUltrasound
view channel.jpeg)
AI-Powered Lung Ultrasound Outperforms Human Experts in Tuberculosis Diagnosis
Despite global declines in tuberculosis (TB) rates in previous years, the incidence of TB rose by 4.6% from 2020 to 2023. Early screening and rapid diagnosis are essential elements of the World Health... Read more
AI Identifies Heart Valve Disease from Common Imaging Test
Tricuspid regurgitation is a condition where the heart's tricuspid valve does not close completely during contraction, leading to backward blood flow, which can result in heart failure. A new artificial... Read moreNuclear Medicine
view channel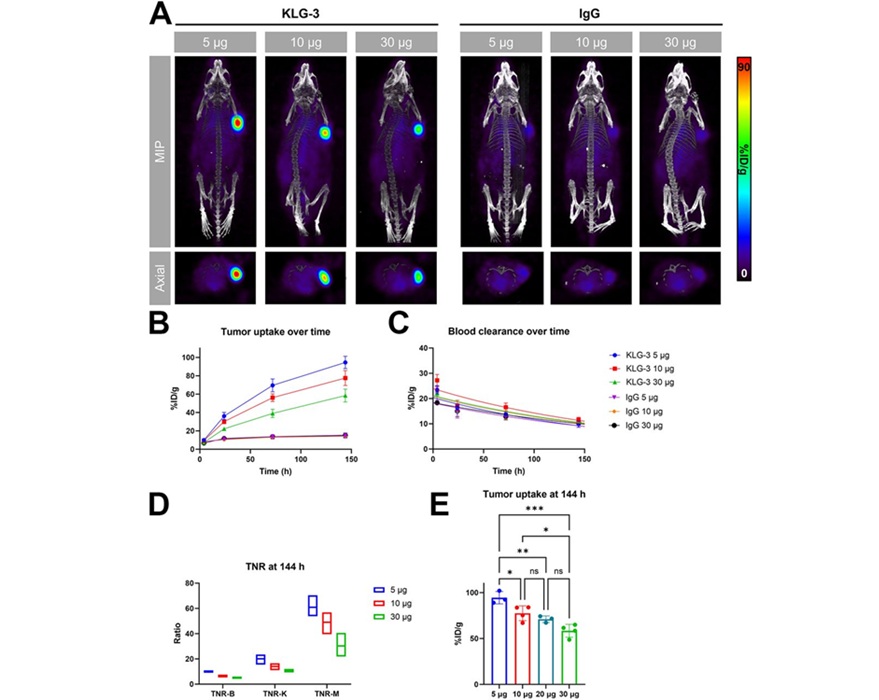
Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
Interleukin-13 receptor α-2 (IL13Rα2) is a cell surface receptor commonly found in solid tumors such as glioblastoma, melanoma, and breast cancer. It is minimally expressed in normal tissues, making it... Read more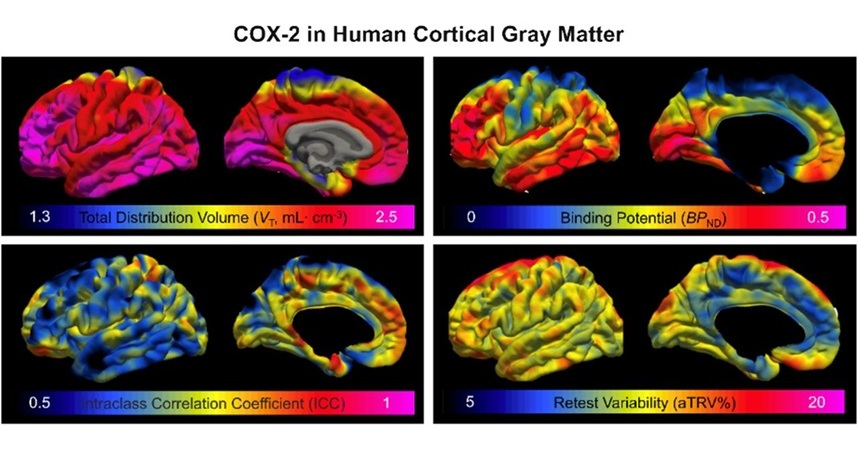
Novel PET Imaging Approach Offers Never-Before-Seen View of Neuroinflammation
COX-2, an enzyme that plays a key role in brain inflammation, can be significantly upregulated by inflammatory stimuli and neuroexcitation. Researchers suggest that COX-2 density in the brain could serve... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel
GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
GE HealthCare (Chicago, IL, USA) has entered into a collaboration with NVIDIA (Santa Clara, CA, USA), expanding the existing relationship between the two companies to focus on pioneering innovation in... Read more
Patient-Specific 3D-Printed Phantoms Transform CT Imaging
New research has highlighted how anatomically precise, patient-specific 3D-printed phantoms are proving to be scalable, cost-effective, and efficient tools in the development of new CT scan algorithms... Read more
Siemens and Sectra Collaborate on Enhancing Radiology Workflows
Siemens Healthineers (Forchheim, Germany) and Sectra (Linköping, Sweden) have entered into a collaboration aimed at enhancing radiologists' diagnostic capabilities and, in turn, improving patient care... Read more