Google Tests AI Algorithm to Help Detect Metastatic Breast Cancers
|
By MedImaging International staff writers Posted on 25 Oct 2018 |
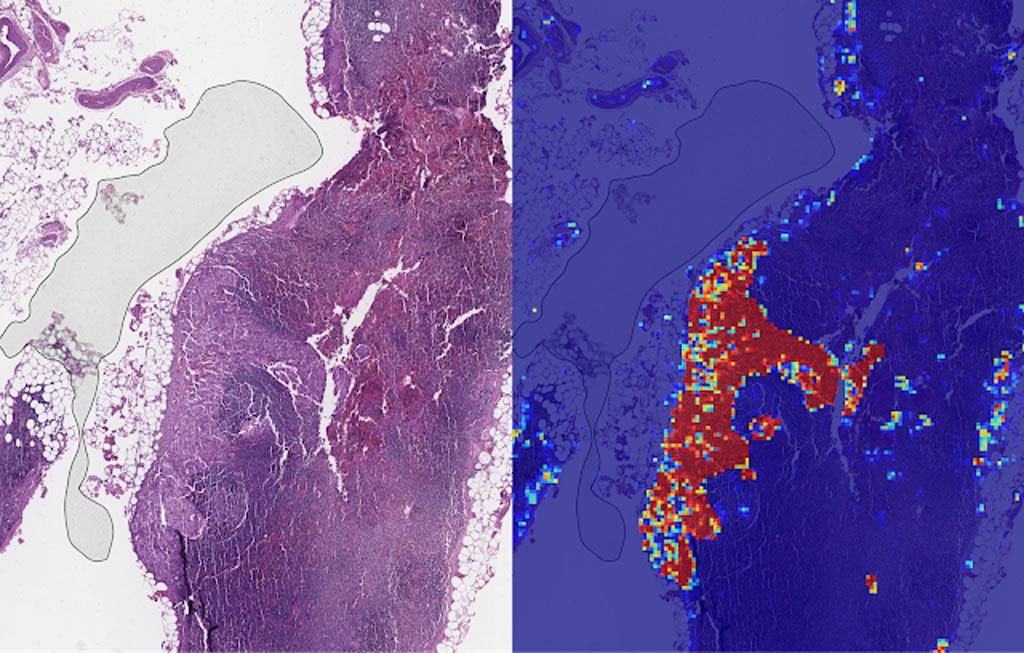
Image: Left: sample view of a slide containing lymph nodes, with multiple artifacts: the dark zone on the left is an air bubble, the white streaks are cutting artifacts, the red hue across some regions are hemorrhagic (containing blood), the tissue is necrotic (decaying), and the processing quality was poor. Right: LYNA identifies the tumor region in the center (red), and correctly classifies the surrounding artifact-laden regions as non-tumor (blue) (Photo courtesy of Google AI).
Scientists at Google AI (Mountain View, CA, USA) who have been developing an algorithm to detect the spread of breast cancer have published new research showing its promise as an assistive tool for pathologists.
Google AI had described its deep learning–based approach to improve diagnostic accuracy (LYmph Node Assistant, or LYNA) to the 2016 ISBI Camelyon Challenge, which provides gigapixel-sized pathology slides of lymph nodes from breast cancer patients for researchers to develop computer algorithms to detect metastatic cancer. LYNA has achieved significantly higher cancer detection rates than had been previously reported. In their latest published studies, the scientists presented a proof-of-concept pathologist assistance tool based on LYNA and their investigation of these factors.
In the first paper, the scientists applied their algorithm to de-identified pathology slides from both the Camelyon Challenge and an independent dataset provided by our co-authors at the Naval Medical Center San Diego. This additional dataset consisted of pathology samples from a different lab using different processes, thereby improving the representation of the diversity of slides and artifacts seen in routine clinical practice. LYNA proved robust to image variability and numerous histological artifacts, and achieved similar performance on both datasets without additional development.
In both the datasets, LYNA was able to correctly distinguish a slide with metastatic cancer from a slide without cancer 99% of the time. Additionally, LYNA could accurately pinpoint the location of both cancers and other suspicious regions within each slide, some of which were too small to be consistently detected by pathologists. Based on this, the researchers believe that one potential benefit of LYNA could be to highlight these areas of concern for pathologists to review and determine the final diagnosis.
In their second paper, six board-certified pathologists completed a simulated diagnostic task in which they reviewed lymph nodes for metastatic breast cancer both with and without the assistance of LYNA. For the often-laborious task of detecting small metastases (termed micrometastases), the use of LYNA made the task subjectively “easier” (according to pathologists’ self-reported diagnostic difficulty) and halved average slide review time, requiring about one minute instead of two minutes per slide.
This indicates the intriguing potential for assistive technologies such as LYNA to reduce the burden of repetitive identification tasks and to allow more time and energy for pathologists to focus on other, more challenging clinical and diagnostic tasks. In terms of diagnostic accuracy, pathologists in this study were able to more reliably detect micrometastases with LYNA, reducing the rate of missed micrometastases by a factor of two. Encouragingly, pathologists with LYNA assistance were more accurate than either unassisted pathologists or the LYNA algorithm itself, indicating that people and algorithms can work together effectively to perform better than working independently.
Related Links:
Google AI
Google AI had described its deep learning–based approach to improve diagnostic accuracy (LYmph Node Assistant, or LYNA) to the 2016 ISBI Camelyon Challenge, which provides gigapixel-sized pathology slides of lymph nodes from breast cancer patients for researchers to develop computer algorithms to detect metastatic cancer. LYNA has achieved significantly higher cancer detection rates than had been previously reported. In their latest published studies, the scientists presented a proof-of-concept pathologist assistance tool based on LYNA and their investigation of these factors.
In the first paper, the scientists applied their algorithm to de-identified pathology slides from both the Camelyon Challenge and an independent dataset provided by our co-authors at the Naval Medical Center San Diego. This additional dataset consisted of pathology samples from a different lab using different processes, thereby improving the representation of the diversity of slides and artifacts seen in routine clinical practice. LYNA proved robust to image variability and numerous histological artifacts, and achieved similar performance on both datasets without additional development.
In both the datasets, LYNA was able to correctly distinguish a slide with metastatic cancer from a slide without cancer 99% of the time. Additionally, LYNA could accurately pinpoint the location of both cancers and other suspicious regions within each slide, some of which were too small to be consistently detected by pathologists. Based on this, the researchers believe that one potential benefit of LYNA could be to highlight these areas of concern for pathologists to review and determine the final diagnosis.
In their second paper, six board-certified pathologists completed a simulated diagnostic task in which they reviewed lymph nodes for metastatic breast cancer both with and without the assistance of LYNA. For the often-laborious task of detecting small metastases (termed micrometastases), the use of LYNA made the task subjectively “easier” (according to pathologists’ self-reported diagnostic difficulty) and halved average slide review time, requiring about one minute instead of two minutes per slide.
This indicates the intriguing potential for assistive technologies such as LYNA to reduce the burden of repetitive identification tasks and to allow more time and energy for pathologists to focus on other, more challenging clinical and diagnostic tasks. In terms of diagnostic accuracy, pathologists in this study were able to more reliably detect micrometastases with LYNA, reducing the rate of missed micrometastases by a factor of two. Encouragingly, pathologists with LYNA assistance were more accurate than either unassisted pathologists or the LYNA algorithm itself, indicating that people and algorithms can work together effectively to perform better than working independently.
Related Links:
Google AI
Latest Industry News News
- GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
- Patient-Specific 3D-Printed Phantoms Transform CT Imaging
- Siemens and Sectra Collaborate on Enhancing Radiology Workflows
- Bracco Diagnostics and ColoWatch Partner to Expand Availability CRC Screening Tests Using Virtual Colonoscopy
- Mindray Partners with TeleRay to Streamline Ultrasound Delivery
- Philips and Medtronic Partner on Stroke Care
- Siemens and Medtronic Enter into Global Partnership for Advancing Spine Care Imaging Technologies
- RSNA 2024 Technical Exhibits to Showcase Latest Advances in Radiology
- Bracco Collaborates with Arrayus on Microbubble-Assisted Focused Ultrasound Therapy for Pancreatic Cancer
- Innovative Collaboration to Enhance Ischemic Stroke Detection and Elevate Standards in Diagnostic Imaging
- RSNA 2024 Registration Opens
- Microsoft collaborates with Leading Academic Medical Systems to Advance AI in Medical Imaging
- GE HealthCare Acquires Intelligent Ultrasound Group’s Clinical Artificial Intelligence Business
- Bayer and Rad AI Collaborate on Expanding Use of Cutting Edge AI Radiology Operational Solutions
- Polish Med-Tech Company BrainScan to Expand Extensively into Foreign Markets
- Hologic Acquires UK-Based Breast Surgical Guidance Company Endomagnetics Ltd.
Channels
Radiography
view channel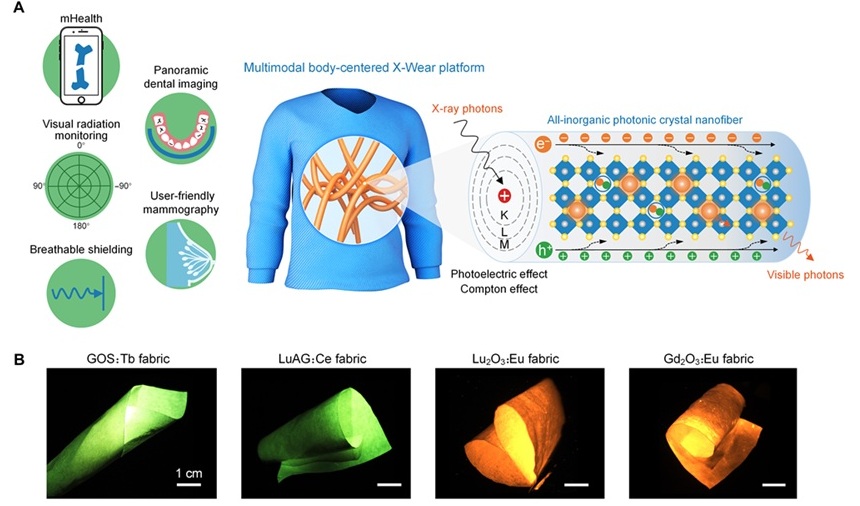
Wearable X-Ray Imaging Detecting Fabric to Provide On-The-Go Diagnostic Scanning
X-rays have been instrumental in modern medical diagnostics since their discovery, from imaging broken bones to screening for early signs of breast cancer. However, traditional X-ray detectors, primarily... Read more
AI Helps Radiologists Spot More Lesions in Mammograms
Breast cancer is a critical health issue, and accurate detection through mammography is essential for effective treatment. However, interpreting mammograms can be challenging for radiologists, particularly... Read moreMRI
view channel
AI Model Outperforms Doctors at Identifying Patients Most At-Risk of Cardiac Arrest
Hypertrophic cardiomyopathy is one of the most common inherited heart conditions and a leading cause of sudden cardiac death in young individuals and athletes. While many patients live normal lives, some... Read more
New MRI Technique Reveals Hidden Heart Issues
Traditional exercise stress tests conducted within an MRI machine require patients to lie flat, a position that artificially improves heart function by increasing stroke volume due to gravity-driven blood... Read moreUltrasound
view channel
Pain-Free Breast Imaging System Performs One Minute Cancer Scan
Breast cancer is one of the leading causes of death for women worldwide, and early detection is key to improving outcomes. Traditional methods like mammograms and ultrasound have their limitations, particularly... Read more
Wireless Chronic Pain Management Device to Reduce Need for Painkillers and Surgery
Chronic pain affects millions of people globally, often leading to long-term disability and dependence on opioid medications, which carry significant risks of side effects and addiction.... Read moreNuclear Medicine
view channel
Novel Bacteria-Specific PET Imaging Approach Detects Hard-To-Diagnose Lung Infections
Mycobacteroides abscessus is a rapidly growing mycobacteria that primarily affects immunocompromised patients and those with underlying lung diseases, such as cystic fibrosis or chronic obstructive pulmonary... Read more
New Imaging Approach Could Reduce Need for Biopsies to Monitor Prostate Cancer
Prostate cancer is the second leading cause of cancer-related death among men in the United States. However, the majority of older men diagnosed with prostate cancer have slow-growing, low-risk forms of... Read moreGeneral/Advanced Imaging
view channel
CT Colonography Beats Stool DNA Testing for Colon Cancer Screening
As colorectal cancer remains the second leading cause of cancer-related deaths worldwide, early detection through screening is vital to reduce advanced-stage treatments and associated costs.... Read more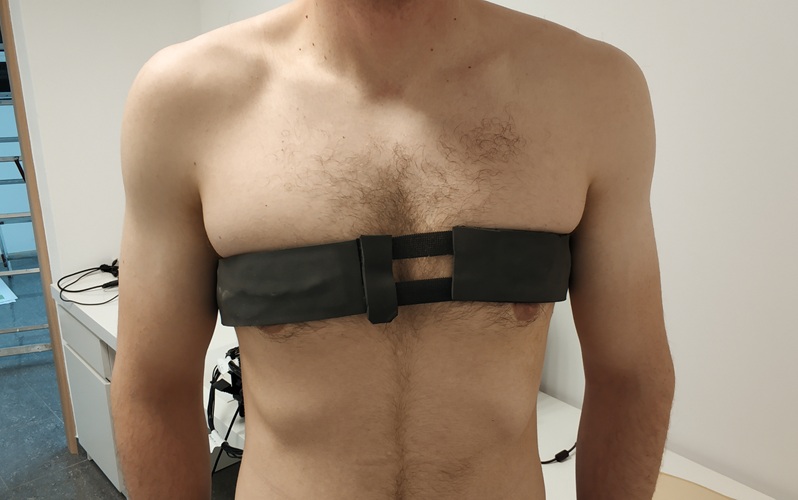
First-Of-Its-Kind Wearable Device Offers Revolutionary Alternative to CT Scans
Currently, patients with conditions such as heart failure, pneumonia, or respiratory distress often require multiple imaging procedures that are intermittent, disruptive, and involve high levels of radiation.... Read more
AI-Based CT Scan Analysis Predicts Early-Stage Kidney Damage Due to Cancer Treatments
Radioligand therapy, a form of targeted nuclear medicine, has recently gained attention for its potential in treating specific types of tumors. However, one of the potential side effects of this therapy... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more