Short-Lived Isotope Opens New Possibilities for Cancer Treatment
|
By MedImaging International staff writers Posted on 08 Sep 2016 |

Image: The SLAC National Accelerator Laboratory (Photo courtesy of SLAC).
A radioisotope of the element actinium is a promising agent for targeted-α therapy to destroy malignant cells, while minimizing the damage to healthy surrounding tissue.
Developed by researchers at Los Alamos National Laboratory (LANL; NM, USA), and in collaboration with the Stanford Linear Accelerator Center (SLAC) Laboratory (SLAC; Menlo Park, CA, USA), targeted-α therapy is based on the radioisotope actinium-225, which has a relatively short half-life of 10 days and emits powerful alpha particles as it decays to stable bismuth. But targeted-α therapy can only become a reliable cancer treatment if actinium securely binds to a chelator, as the radioisotope is very toxic to healthy tissue.
In an attempt to clarify the limited understanding of actinium chemistry, the researchers used X-ray absorption spectroscopy and molecular dynamics density functional theory to investigate actinium coordination chemistry. They were able to determine information about chemical bonds formed by actinium, including what binds to the element, how many atoms are present, and the distances between them. The researchers will now attempt to engineer a chelator carrier molecule that could safely transport actinium-225 through the body to tumor cells. The study was published on August 17, 2016, in Nature Communications.
“Imagine if someone gave you the element iron and nothing was known. That’s almost the same place we were in with actinium, as far as macroscopic chemistry goes. The Manhattan Project scientists used film to see the X-rays released off the sample,” said study co-author Stosh Kozimor, PhD, an isotope chemist at Los Alamos, “but the radioactivity from actinium darkened the film before they could make meaningful measurements. They were only able to get a fingerprint of the actinium compounds that were suggestive of what formed. Beyond that, there isn’t much information in those measurements.”
Actinium (Ac), discovered in 1899, is a radioactive chemical element with the atomic number 89. It was the first non-primordial radioactive element to be isolated. A soft, silvery-white metal, it reacts rapidly with oxygen and moisture in air, forming a white coating of actinium oxide that prevents further oxidation. One ton of natural uranium in ore contains about 0.2 milligrams of actinium-227. Owing to its scarcity, high price, and radioactivity, actinium has no significant industrial use.
Related Links:
Los Alamos National Laboratory
Stanford Linear Accelerator Center Laboratory
Developed by researchers at Los Alamos National Laboratory (LANL; NM, USA), and in collaboration with the Stanford Linear Accelerator Center (SLAC) Laboratory (SLAC; Menlo Park, CA, USA), targeted-α therapy is based on the radioisotope actinium-225, which has a relatively short half-life of 10 days and emits powerful alpha particles as it decays to stable bismuth. But targeted-α therapy can only become a reliable cancer treatment if actinium securely binds to a chelator, as the radioisotope is very toxic to healthy tissue.
In an attempt to clarify the limited understanding of actinium chemistry, the researchers used X-ray absorption spectroscopy and molecular dynamics density functional theory to investigate actinium coordination chemistry. They were able to determine information about chemical bonds formed by actinium, including what binds to the element, how many atoms are present, and the distances between them. The researchers will now attempt to engineer a chelator carrier molecule that could safely transport actinium-225 through the body to tumor cells. The study was published on August 17, 2016, in Nature Communications.
“Imagine if someone gave you the element iron and nothing was known. That’s almost the same place we were in with actinium, as far as macroscopic chemistry goes. The Manhattan Project scientists used film to see the X-rays released off the sample,” said study co-author Stosh Kozimor, PhD, an isotope chemist at Los Alamos, “but the radioactivity from actinium darkened the film before they could make meaningful measurements. They were only able to get a fingerprint of the actinium compounds that were suggestive of what formed. Beyond that, there isn’t much information in those measurements.”
Actinium (Ac), discovered in 1899, is a radioactive chemical element with the atomic number 89. It was the first non-primordial radioactive element to be isolated. A soft, silvery-white metal, it reacts rapidly with oxygen and moisture in air, forming a white coating of actinium oxide that prevents further oxidation. One ton of natural uranium in ore contains about 0.2 milligrams of actinium-227. Owing to its scarcity, high price, and radioactivity, actinium has no significant industrial use.
Related Links:
Los Alamos National Laboratory
Stanford Linear Accelerator Center Laboratory
Latest Nuclear Medicine News
- New PET Biomarker Predicts Success of Immune Checkpoint Blockade Therapy
- New PET Agent Rapidly and Accurately Visualizes Lesions in Clear Cell Renal Cell Carcinoma Patients
- New Imaging Technique Monitors Inflammation Disorders without Radiation Exposure
- New SPECT/CT Technique Could Change Imaging Practices and Increase Patient Access
- New Radiotheranostic System Detects and Treats Ovarian Cancer Noninvasively
- AI System Automatically and Reliably Detects Cardiac Amyloidosis Using Scintigraphy Imaging
- Early 30-Minute Dynamic FDG-PET Acquisition Could Halve Lung Scan Times
- New Method for Triggering and Imaging Seizures to Help Guide Epilepsy Surgery
- Radioguided Surgery Accurately Detects and Removes Metastatic Lymph Nodes in Prostate Cancer Patients
- New PET Tracer Detects Inflammatory Arthritis Before Symptoms Appear
- Novel PET Tracer Enhances Lesion Detection in Medullary Thyroid Cancer
- Targeted Therapy Delivers Radiation Directly To Cells in Hard-To-Treat Cancers
- New PET Tracer Noninvasively Identifies Cancer Gene Mutation for More Precise Diagnosis
- Algorithm Predicts Prostate Cancer Recurrence in Patients Treated by Radiation Therapy
- Novel PET Imaging Tracer Noninvasively Identifies Cancer Gene Mutation for More Precise Diagnosis
- Ultrafast Laser Technology to Improve Cancer Treatment
Channels
Radiography
view channel
Novel Breast Imaging System Proves As Effective As Mammography
Breast cancer remains the most frequently diagnosed cancer among women. It is projected that one in eight women will be diagnosed with breast cancer during her lifetime, and one in 42 women who turn 50... Read more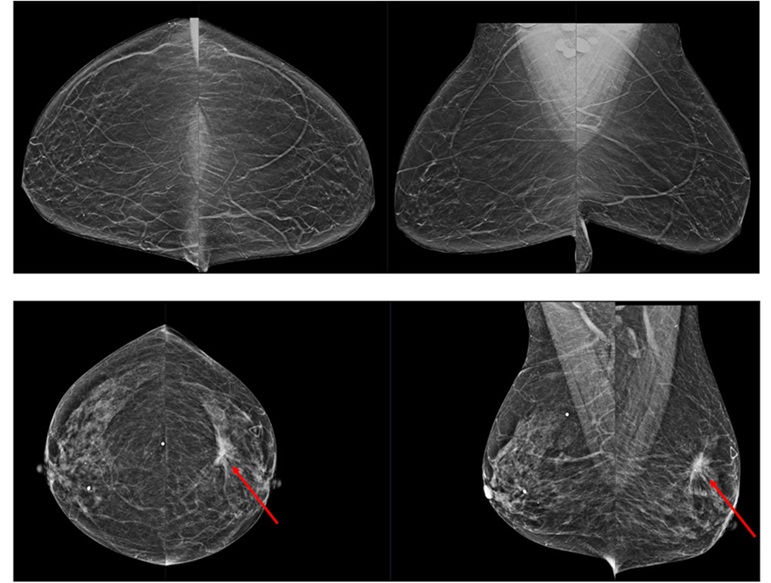
AI Assistance Improves Breast-Cancer Screening by Reducing False Positives
Radiologists typically detect one case of cancer for every 200 mammograms reviewed. However, these evaluations often result in false positives, leading to unnecessary patient recalls for additional testing,... Read moreMRI
view channel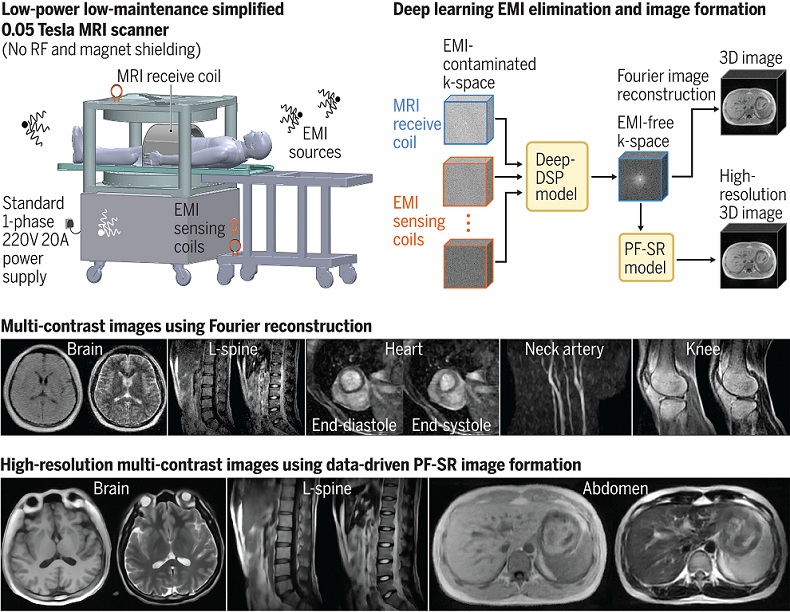
Low-Cost Whole-Body MRI Device Combined with AI Generates High-Quality Results
Magnetic Resonance Imaging (MRI) has significantly transformed healthcare, providing a noninvasive, radiation-free method for detailed imaging. It is especially promising for the future of medical diagnosis... Read more
World's First Whole-Body Ultra-High Field MRI Officially Comes To Market
The world's first whole-body ultra-high field (UHF) MRI has officially come to market, marking a remarkable advancement in diagnostic radiology. United Imaging (Shanghai, China) has secured clearance from the U.... Read moreUltrasound
view channel.jpg)
Diagnostic System Automatically Analyzes TTE Images to Identify Congenital Heart Disease
Congenital heart disease (CHD) is one of the most prevalent congenital anomalies worldwide, presenting substantial health and financial challenges for affected patients. Early detection and treatment of... Read more
Super-Resolution Imaging Technique Could Improve Evaluation of Cardiac Conditions
The heart depends on efficient blood circulation to pump blood throughout the body, delivering oxygen to tissues and removing carbon dioxide and waste. Yet, when heart vessels are damaged, it can disrupt... Read more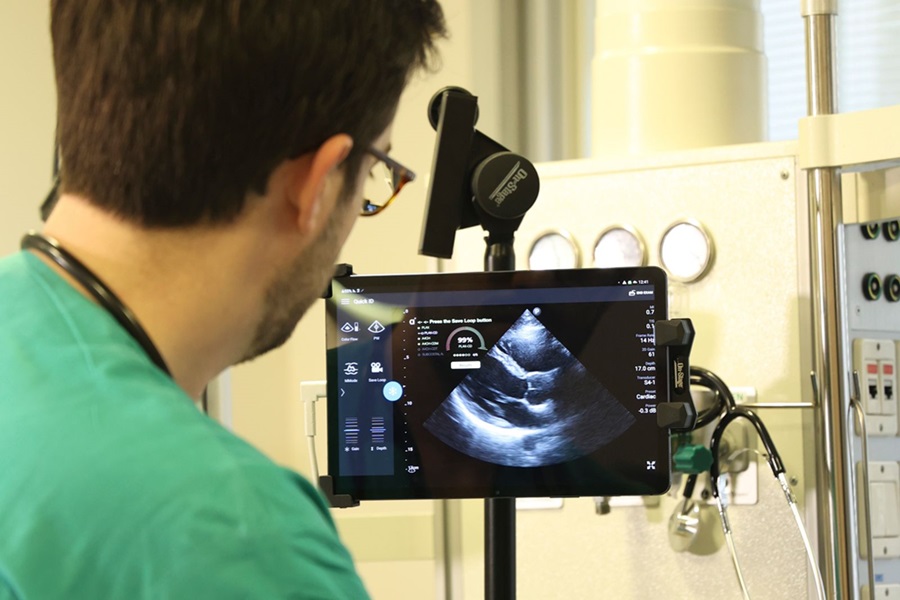
First AI-Powered POC Ultrasound Diagnostic Solution Helps Prioritize Cases Based On Severity
Ultrasound scans are essential for identifying and diagnosing various medical conditions, but often, patients must wait weeks or months for results due to a shortage of qualified medical professionals... Read moreGeneral/Advanced Imaging
view channelBone Density Test Uses Existing CT Images to Predict Fractures
Osteoporotic fractures are not only devastating and deadly, especially hip fractures, but also impose significant costs. They rank among the top chronic diseases in terms of disability-adjusted life years... Read more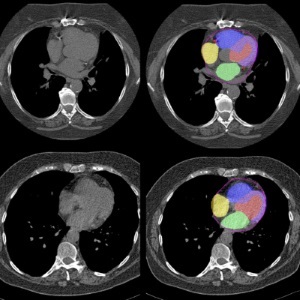
AI Predicts Cardiac Risk and Mortality from Routine Chest CT Scans
Heart disease remains the leading cause of death and is largely preventable, yet many individuals are unaware of their risk until it becomes severe. Early detection through screening can reveal heart issues,... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel
Hologic Acquires UK-Based Breast Surgical Guidance Company Endomagnetics Ltd.
Hologic, Inc. (Marlborough, MA, USA) has entered into a definitive agreement to acquire Endomagnetics Ltd. (Cambridge, UK), a privately held developer of breast cancer surgery technologies, for approximately... Read more
Bayer and Google Partner on New AI Product for Radiologists
Medical imaging data comprises around 90% of all healthcare data, and it is a highly complex and rich clinical data modality and serves as a vital tool for diagnosing patients. Each year, billions of medical... Read more