US FDA Clears Smart Mobile-Connected High-Resolution Bladder Scanner
|
By MedImaging International staff writers Posted on 18 May 2016 |
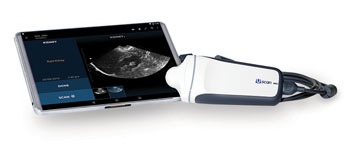
Image: The Uscan smart mobile-connected high-resolution bladder scanner is now cleared by the US FDA (Photo courtesy of Signostics).
A global medical device manufacturer has received US FDA clearance for a new urologic visualization device that can improve point-of-care clinical decision-making.
The device is the first mobile-connected ultrasound visualization device targeted at urologic care to receive US Food and Drug Administration (FDA) 510(k) clearance.
The Uscan device developed by Signostics (Bothell, WA, USA) has a removable probe, a high-resolution tablet with a touch screen, and handheld displays. The Uscan is compatible with the Android operating system, features built-in WiFi and Bluetooth connectivity, and is interoperable with Electronic Health Record (EHR) systems. The device does not require annual calibration, and provides real-time user guidance.
The Uscan can acquire up to 256 bladder slices, and uses algorithms to actively recognize bladder contours in 3D providing accurate volume measurements. The device can also be used for real-time ultrasound imaging of the bladder stones, gallbladder, prostate, pelvic floor, kidneys, for the placement of catheters, and for rapid visual tracking, and observation. The device can also be used in emergency departments, maternity wards, for pediatrics, oncology, rehabilitation, in home nursing, and in geriatrics.
CEO of Signostics, Kevin Goodwin, said, “Uscan doesn’t just scan; it sees – providing intelligent urologic visualization by leveraging science from current-day computer vision algorithms aimed at more efficient and confident point-of-care clinical decision-making. Uscan will exceed historical industry standards for bladder volume measurement accuracy yet will also enable use for other urologic imaging needs, reducing the delays and expense of engaging specialized ultrasound equipment or sonographers.”
Related Links:
Signostics
The device is the first mobile-connected ultrasound visualization device targeted at urologic care to receive US Food and Drug Administration (FDA) 510(k) clearance.
The Uscan device developed by Signostics (Bothell, WA, USA) has a removable probe, a high-resolution tablet with a touch screen, and handheld displays. The Uscan is compatible with the Android operating system, features built-in WiFi and Bluetooth connectivity, and is interoperable with Electronic Health Record (EHR) systems. The device does not require annual calibration, and provides real-time user guidance.
The Uscan can acquire up to 256 bladder slices, and uses algorithms to actively recognize bladder contours in 3D providing accurate volume measurements. The device can also be used for real-time ultrasound imaging of the bladder stones, gallbladder, prostate, pelvic floor, kidneys, for the placement of catheters, and for rapid visual tracking, and observation. The device can also be used in emergency departments, maternity wards, for pediatrics, oncology, rehabilitation, in home nursing, and in geriatrics.
CEO of Signostics, Kevin Goodwin, said, “Uscan doesn’t just scan; it sees – providing intelligent urologic visualization by leveraging science from current-day computer vision algorithms aimed at more efficient and confident point-of-care clinical decision-making. Uscan will exceed historical industry standards for bladder volume measurement accuracy yet will also enable use for other urologic imaging needs, reducing the delays and expense of engaging specialized ultrasound equipment or sonographers.”
Related Links:
Signostics
Latest Imaging IT News
- New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
- Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
- AI-Based Mammography Triage Software Helps Dramatically Improve Interpretation Process
- Artificial Intelligence (AI) Program Accurately Predicts Lung Cancer Risk from CT Images
- Image Management Platform Streamlines Treatment Plans
- AI-Based Technology for Ultrasound Image Analysis Receives FDA Approval
- AI Technology for Detecting Breast Cancer Receives CE Mark Approval
- Digital Pathology Software Improves Workflow Efficiency
- Patient-Centric Portal Facilitates Direct Imaging Access
- New Workstation Supports Customer-Driven Imaging Workflow
Channels
Radiography
view channel
Novel Breast Imaging System Proves As Effective As Mammography
Breast cancer remains the most frequently diagnosed cancer among women. It is projected that one in eight women will be diagnosed with breast cancer during her lifetime, and one in 42 women who turn 50... Read more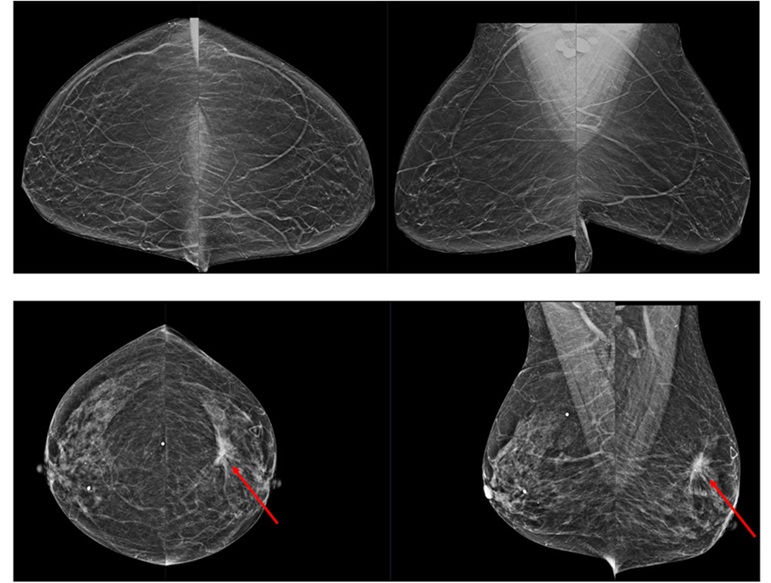
AI Assistance Improves Breast-Cancer Screening by Reducing False Positives
Radiologists typically detect one case of cancer for every 200 mammograms reviewed. However, these evaluations often result in false positives, leading to unnecessary patient recalls for additional testing,... Read moreMRI
view channel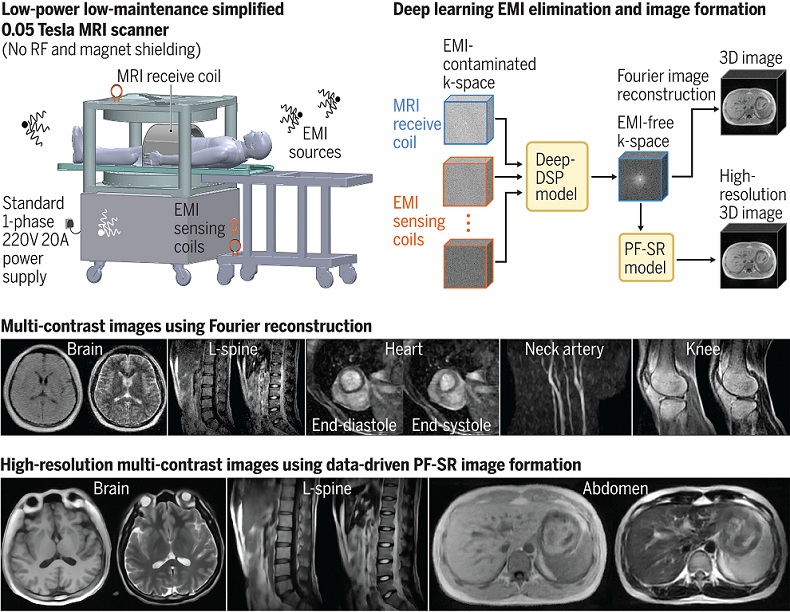
Low-Cost Whole-Body MRI Device Combined with AI Generates High-Quality Results
Magnetic Resonance Imaging (MRI) has significantly transformed healthcare, providing a noninvasive, radiation-free method for detailed imaging. It is especially promising for the future of medical diagnosis... Read more
World's First Whole-Body Ultra-High Field MRI Officially Comes To Market
The world's first whole-body ultra-high field (UHF) MRI has officially come to market, marking a remarkable advancement in diagnostic radiology. United Imaging (Shanghai, China) has secured clearance from the U.... Read moreNuclear Medicine
view channel
New PET Biomarker Predicts Success of Immune Checkpoint Blockade Therapy
Immunotherapies, such as immune checkpoint blockade (ICB), have shown promising clinical results in treating melanoma, non-small cell lung cancer, and other tumor types. However, the effectiveness of these... Read moreNew PET Agent Rapidly and Accurately Visualizes Lesions in Clear Cell Renal Cell Carcinoma Patients
Clear cell renal cell cancer (ccRCC) represents 70-80% of renal cell carcinoma cases. While localized disease can be effectively treated with surgery and ablative therapies, one-third of patients either... Read more
New Imaging Technique Monitors Inflammation Disorders without Radiation Exposure
Imaging inflammation using traditional radiological techniques presents significant challenges, including radiation exposure, poor image quality, high costs, and invasive procedures. Now, new contrast... Read more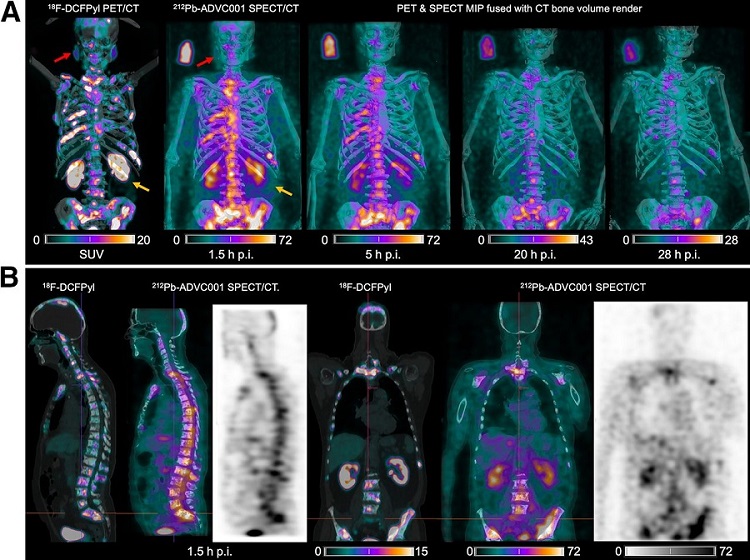
New SPECT/CT Technique Could Change Imaging Practices and Increase Patient Access
The development of lead-212 (212Pb)-PSMA–based targeted alpha therapy (TAT) is garnering significant interest in treating patients with metastatic castration-resistant prostate cancer. The imaging of 212Pb,... Read moreGeneral/Advanced Imaging
view channelBone Density Test Uses Existing CT Images to Predict Fractures
Osteoporotic fractures are not only devastating and deadly, especially hip fractures, but also impose significant costs. They rank among the top chronic diseases in terms of disability-adjusted life years... Read more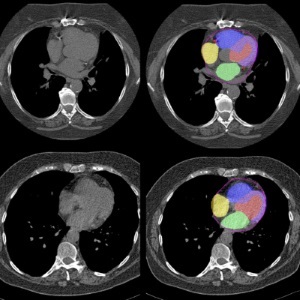
AI Predicts Cardiac Risk and Mortality from Routine Chest CT Scans
Heart disease remains the leading cause of death and is largely preventable, yet many individuals are unaware of their risk until it becomes severe. Early detection through screening can reveal heart issues,... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel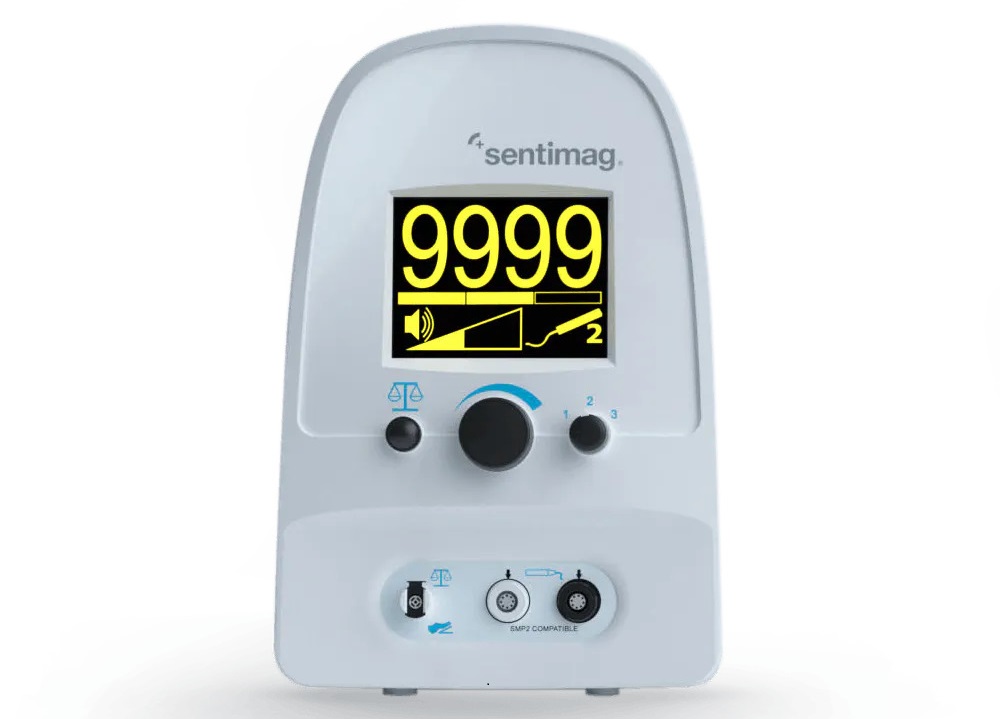
Hologic Acquires UK-Based Breast Surgical Guidance Company Endomagnetics Ltd.
Hologic, Inc. (Marlborough, MA, USA) has entered into a definitive agreement to acquire Endomagnetics Ltd. (Cambridge, UK), a privately held developer of breast cancer surgery technologies, for approximately... Read more
Bayer and Google Partner on New AI Product for Radiologists
Medical imaging data comprises around 90% of all healthcare data, and it is a highly complex and rich clinical data modality and serves as a vital tool for diagnosing patients. Each year, billions of medical... Read more