Cancer Detection Technology Built on AI Receives FDA Clearance
|
By MedImaging International staff writers Posted on 25 Dec 2018 |
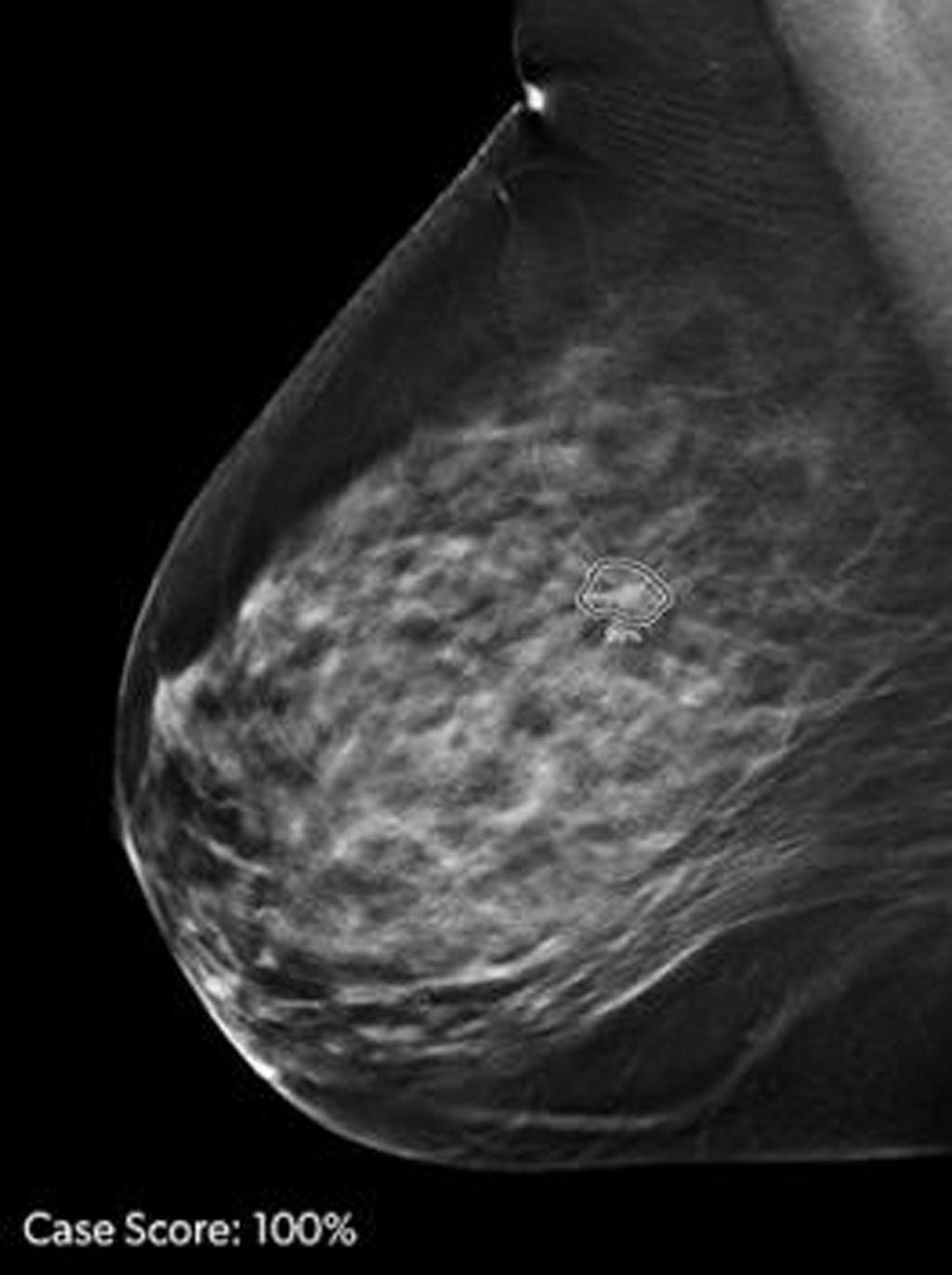
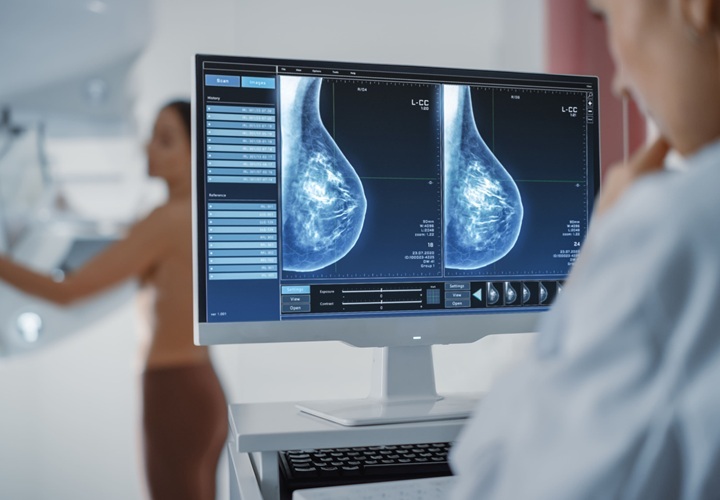
Image: ProFound AI is a deep-learning cancer detection and workflow solution for DBT (Photo courtesy of iCAD).
A new deep-learning cancer detection software solution for digital breast tomosynthesis (DBT) has received clearance from the US FDA for commercial sales and clinical use in US. The solution named ProFound AI is built on artificial intelligence (AI) and has been developed by iCAD, Inc (Nashua, NH, USA), a provider of cancer detection and therapy solutions.
ProFound AI is a deep-learning cancer detection and workflow solution for DBT that delivers critical benefits to radiologists, their facilities, and their patients through an improvement in cancer detection rates by an average of 8% and reduction in unnecessary patient recall rates by an average of 7%. The technology is trained to detect malignant soft-tissue densities and calcifications. It also provides radiologists with scoring information representing the likelihood that a detection or case is malignant based on the large dataset of clinical images used to train the algorithm.
ProFound AI has received FDA clearance based on positive clinical results from a large reader study completed earlier this year and presented at this year’s Radiological Society of North America (RSNA) annual meeting in Chicago, USA. In the study, 24 radiologists read 260 tomosynthesis cases, both with and without the ProFound AI solution. The study’s findings revealed increased cancer detection rates, reduced false positive rates and patient recalls, and a significant decrease of more than 50% on average in interpretation times.
“Obtaining FDA clearance for ProFound AI opens a new and substantial addressable market for iCAD. This enables us to offer clinicians globally an unrivaled cancer detection and workflow solution built on the latest advances in deep-learning,” said Stacey Stevens, Executive Vice President and Chief Strategy and Commercial Officer at iCAD. “Clinical reader study results and comprehensive stand-alone testing have shown unprecedented improvements in both clinical performance and reading efficiency. We are proud to introduce revolutionary technology that will fundamentally transform breast cancer detection and patient care.”
ProFound AI is a deep-learning cancer detection and workflow solution for DBT that delivers critical benefits to radiologists, their facilities, and their patients through an improvement in cancer detection rates by an average of 8% and reduction in unnecessary patient recall rates by an average of 7%. The technology is trained to detect malignant soft-tissue densities and calcifications. It also provides radiologists with scoring information representing the likelihood that a detection or case is malignant based on the large dataset of clinical images used to train the algorithm.
ProFound AI has received FDA clearance based on positive clinical results from a large reader study completed earlier this year and presented at this year’s Radiological Society of North America (RSNA) annual meeting in Chicago, USA. In the study, 24 radiologists read 260 tomosynthesis cases, both with and without the ProFound AI solution. The study’s findings revealed increased cancer detection rates, reduced false positive rates and patient recalls, and a significant decrease of more than 50% on average in interpretation times.
“Obtaining FDA clearance for ProFound AI opens a new and substantial addressable market for iCAD. This enables us to offer clinicians globally an unrivaled cancer detection and workflow solution built on the latest advances in deep-learning,” said Stacey Stevens, Executive Vice President and Chief Strategy and Commercial Officer at iCAD. “Clinical reader study results and comprehensive stand-alone testing have shown unprecedented improvements in both clinical performance and reading efficiency. We are proud to introduce revolutionary technology that will fundamentally transform breast cancer detection and patient care.”
Latest Industry News News
- GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
- Patient-Specific 3D-Printed Phantoms Transform CT Imaging
- Siemens and Sectra Collaborate on Enhancing Radiology Workflows
- Bracco Diagnostics and ColoWatch Partner to Expand Availability CRC Screening Tests Using Virtual Colonoscopy
- Mindray Partners with TeleRay to Streamline Ultrasound Delivery
- Philips and Medtronic Partner on Stroke Care
- Siemens and Medtronic Enter into Global Partnership for Advancing Spine Care Imaging Technologies
- RSNA 2024 Technical Exhibits to Showcase Latest Advances in Radiology
- Bracco Collaborates with Arrayus on Microbubble-Assisted Focused Ultrasound Therapy for Pancreatic Cancer
- Innovative Collaboration to Enhance Ischemic Stroke Detection and Elevate Standards in Diagnostic Imaging
- RSNA 2024 Registration Opens
- Microsoft collaborates with Leading Academic Medical Systems to Advance AI in Medical Imaging
- GE HealthCare Acquires Intelligent Ultrasound Group’s Clinical Artificial Intelligence Business
- Bayer and Rad AI Collaborate on Expanding Use of Cutting Edge AI Radiology Operational Solutions
- Polish Med-Tech Company BrainScan to Expand Extensively into Foreign Markets
- Hologic Acquires UK-Based Breast Surgical Guidance Company Endomagnetics Ltd.
Channels
Radiography
view channel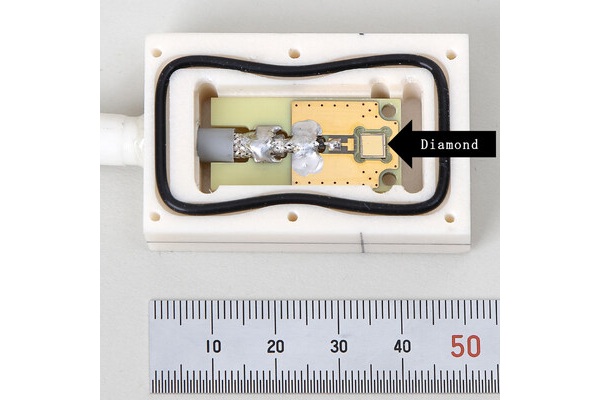
World's Largest Class Single Crystal Diamond Radiation Detector Opens New Possibilities for Diagnostic Imaging
Diamonds possess ideal physical properties for radiation detection, such as exceptional thermal and chemical stability along with a quick response time. Made of carbon with an atomic number of six, diamonds... Read more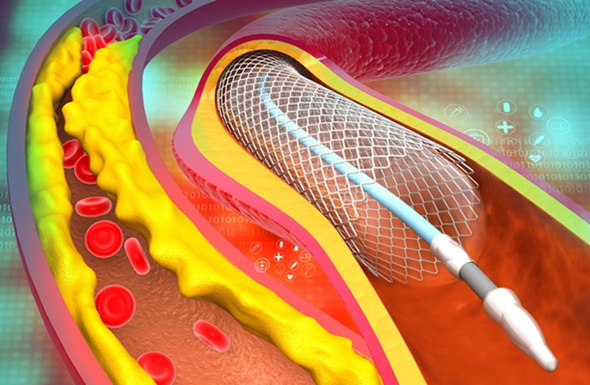
AI-Powered Imaging Technique Shows Promise in Evaluating Patients for PCI
Percutaneous coronary intervention (PCI), also known as coronary angioplasty, is a minimally invasive procedure where small metal tubes called stents are inserted into partially blocked coronary arteries... Read moreMRI
view channel
AI Tool Predicts Relapse of Pediatric Brain Cancer from Brain MRI Scans
Many pediatric gliomas are treatable with surgery alone, but relapses can be catastrophic. Predicting which patients are at risk for recurrence remains challenging, leading to frequent follow-ups with... Read more
AI Tool Tracks Effectiveness of Multiple Sclerosis Treatments Using Brain MRI Scans
Multiple sclerosis (MS) is a condition in which the immune system attacks the brain and spinal cord, leading to impairments in movement, sensation, and cognition. Magnetic Resonance Imaging (MRI) markers... Read more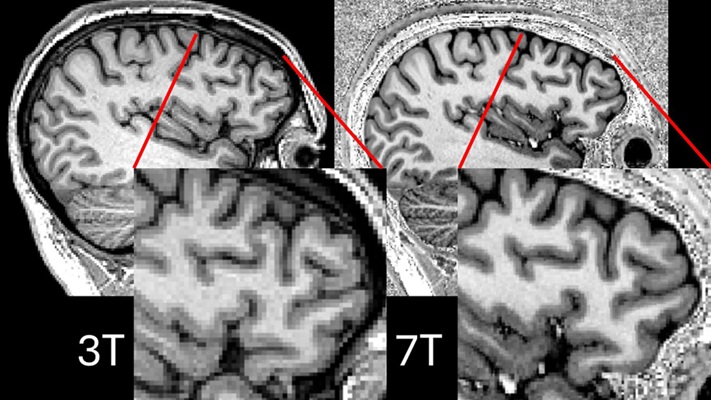
Ultra-Powerful MRI Scans Enable Life-Changing Surgery in Treatment-Resistant Epileptic Patients
Approximately 360,000 individuals in the UK suffer from focal epilepsy, a condition in which seizures spread from one part of the brain. Around a third of these patients experience persistent seizures... Read moreUltrasound
view channel.jpeg)
AI-Powered Lung Ultrasound Outperforms Human Experts in Tuberculosis Diagnosis
Despite global declines in tuberculosis (TB) rates in previous years, the incidence of TB rose by 4.6% from 2020 to 2023. Early screening and rapid diagnosis are essential elements of the World Health... Read more
AI Identifies Heart Valve Disease from Common Imaging Test
Tricuspid regurgitation is a condition where the heart's tricuspid valve does not close completely during contraction, leading to backward blood flow, which can result in heart failure. A new artificial... Read moreNuclear Medicine
view channel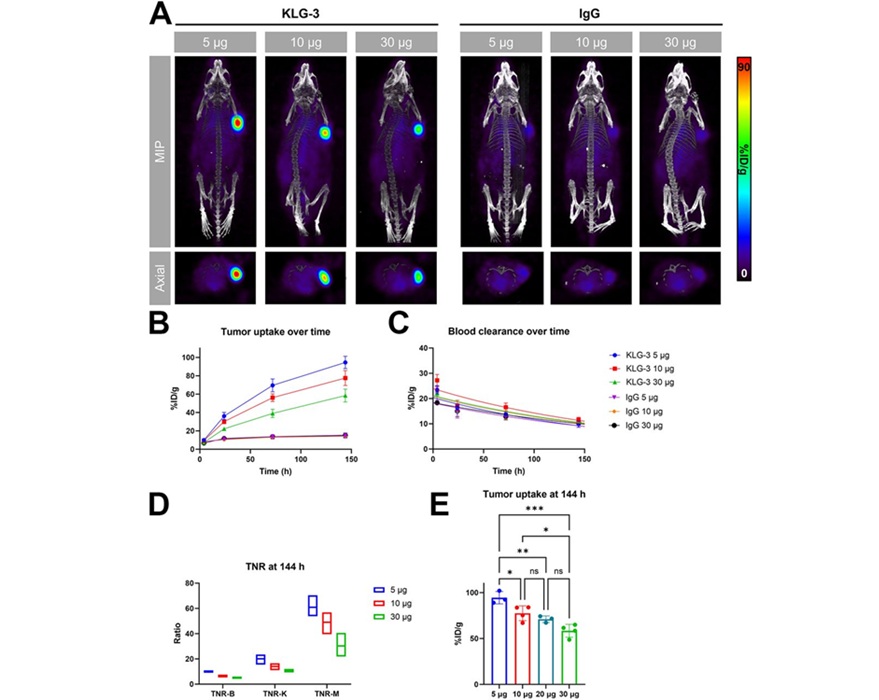
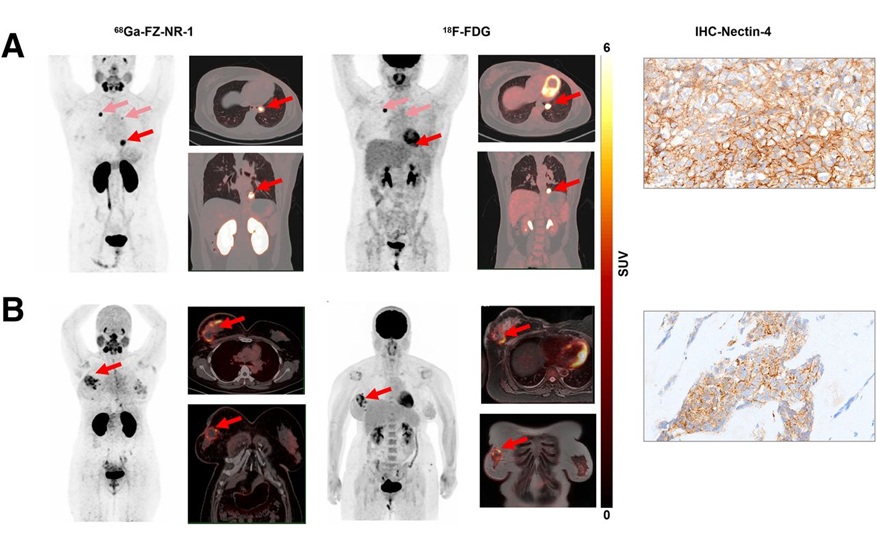
Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
Interleukin-13 receptor α-2 (IL13Rα2) is a cell surface receptor commonly found in solid tumors such as glioblastoma, melanoma, and breast cancer. It is minimally expressed in normal tissues, making it... Read more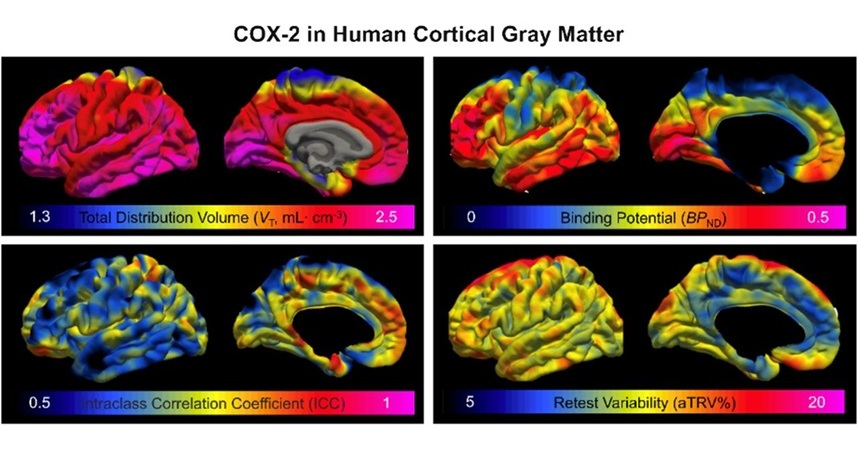
Novel PET Imaging Approach Offers Never-Before-Seen View of Neuroinflammation
COX-2, an enzyme that plays a key role in brain inflammation, can be significantly upregulated by inflammatory stimuli and neuroexcitation. Researchers suggest that COX-2 density in the brain could serve... Read moreGeneral/Advanced Imaging
view channel
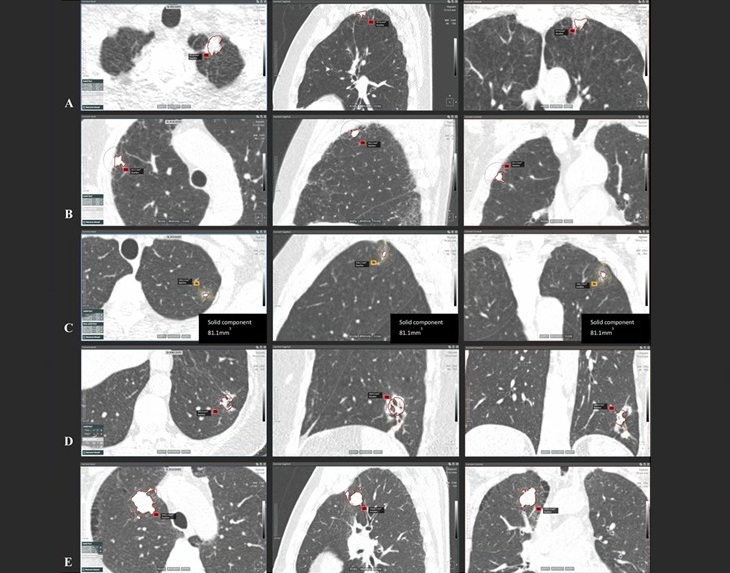
AI-Powered Imaging System Improves Lung Cancer Diagnosis
Given the need to detect lung cancer at earlier stages, there is an increasing need for a definitive diagnostic pathway for patients with suspicious pulmonary nodules. However, obtaining tissue samples... Read more
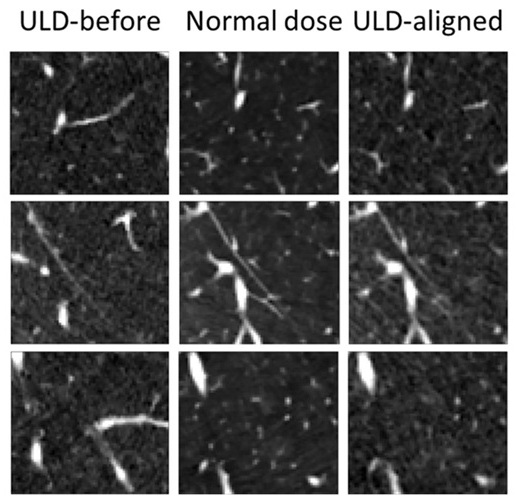
AI Model Significantly Enhances Low-Dose CT Capabilities
Lung cancer remains one of the most challenging diseases, making early diagnosis vital for effective treatment. Fortunately, advancements in artificial intelligence (AI) are revolutionizing lung cancer... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more