Enhanced PET Imaging Radiotracers Designed for Better Tracking of Disease
|
By MedImaging International staff writers Posted on 09 Jun 2014 |
Scientists have developed a direct approach for making single enantiomer positron emission tomography (PET) tracers.
Small molecules containing a radioactive isotope of fluorine called 18F radiotracers are used to detect and track specific diseases in patients. When injected into the body, these molecules collect in specific targets, such as tumors, and can be visualized by their radioactive tag on a PET imaging scan. The 18F tags rapidly decay, therefore, no radioactivity remains after approximately one day.
But there are only a few strategies available for making 18F radiotracers. Furthermore, existing techniques tend to require harsh conditions that jumble the placement of a radiotracer’s more delicate chemical bonds. Researchers at Princeton University (Princeton, NJ, USA) reported that they now have a way to produce 18F radiotracers that avoids that problem. “It’s the first method to do enantio-selective carbon-18F bond formation,” said lead investigator Dr. Abigail Doyle, a Princeton associate professor of chemistry.
Radiotracers up to now have mostly been evaluated as mixtures of enantiomers. Enantiomers are molecules that are totally identical in composition but the arrangement of atoms at the chiral center are mirror images. A chiral center is an atom, typically carbon, which is connected to four different groups. “We know in biology, small molecule interactions with enzymes often depend on the 3D [three-dimensional] properties of the molecule. Being able to prepare the enantiomers of a given chiral tracer, in order to optimize which tracer has the best binding and imaging properties could be really useful,” Dr. Doyle said.
The researchers developed a cobalt fluoride catalyst—[18F](salen)CoF—to install the radioactive fluoride through the ring-opening reaction of epoxides. Their approach demonstrated excellent enantioselectivity for 11 substrates, five of which are known pre-clinical PET tracers. With this new method, researchers can now assess single enantiomers of existing or new PET radiotracers and evaluate if these compounds offer any benefits over the enantiomeric combinations. Eventually, the goal is to use this chemistry to identify a completely new PET radiotracer for imaging.
Currently, there are only four US Food and Drug Administration (FDA)-approved 18F radiotracers. One of the major limitations to discovering PET tracers is the fact that the only commercially available source of 18F is nucleophilic fluoride. Existing 18F sources are very basic, and during the process of making the 18F radiotracer, can cause the elimination of alcohol and amine groups and rearrange the groups around a chiral center in a process called racemization. Under Dr. Doyle’s less basic reaction conditions, even alcohols and secondary amines are tolerated and no racemization is seen.
“Forming carbon-fluorine bonds by nucleophilic fluoride is challenging. One typically needs to use high temperatures or else the reactions are too slow to permit radioisotope incorporation,” Dr. Doyle commented. “Whereas most reactions require temperatures greater than 100° Celsius, our reaction can be run at 50° Celsius.”
Small amounts of radioactivity were sufficient to develop the reaction at first but to perform imaging studies, larger amounts of radioactivity are necessary. “When you go to higher activity, that’s when you do automated chemistry in a hot cell, which is basically a block of lead so you get no exposure,” Dr. Doyle said.
To be efficient in an industrial environment, the chemistry needs to be converted from the laboratory to an automated hot cell. The researchers were given access to an automated hot cell nearby at Merck’s West Point (PA, USA) site. The whole process of radiolabeling takes about 30 to 45 minutes when it is automated. The set-up includes a robotic arm that delivers solutions to designated vials, a high-performance liquid chromatography (HPLC) system and a rotary evaporator, which are devices for the analysis and purification of the radiotracers.
“The catalyst is very robust and the fact that we can translate the reaction directly to the hot cell bodes very well for non-experts to be able to run these sorts of reactions,” Dr. Doyle concluded. “We demonstrated that the radioactivity is high enough that we could actually use it for imaging. That’s an exciting next step.”
Related Links:
Princeton University
Small molecules containing a radioactive isotope of fluorine called 18F radiotracers are used to detect and track specific diseases in patients. When injected into the body, these molecules collect in specific targets, such as tumors, and can be visualized by their radioactive tag on a PET imaging scan. The 18F tags rapidly decay, therefore, no radioactivity remains after approximately one day.
But there are only a few strategies available for making 18F radiotracers. Furthermore, existing techniques tend to require harsh conditions that jumble the placement of a radiotracer’s more delicate chemical bonds. Researchers at Princeton University (Princeton, NJ, USA) reported that they now have a way to produce 18F radiotracers that avoids that problem. “It’s the first method to do enantio-selective carbon-18F bond formation,” said lead investigator Dr. Abigail Doyle, a Princeton associate professor of chemistry.
Radiotracers up to now have mostly been evaluated as mixtures of enantiomers. Enantiomers are molecules that are totally identical in composition but the arrangement of atoms at the chiral center are mirror images. A chiral center is an atom, typically carbon, which is connected to four different groups. “We know in biology, small molecule interactions with enzymes often depend on the 3D [three-dimensional] properties of the molecule. Being able to prepare the enantiomers of a given chiral tracer, in order to optimize which tracer has the best binding and imaging properties could be really useful,” Dr. Doyle said.
The researchers developed a cobalt fluoride catalyst—[18F](salen)CoF—to install the radioactive fluoride through the ring-opening reaction of epoxides. Their approach demonstrated excellent enantioselectivity for 11 substrates, five of which are known pre-clinical PET tracers. With this new method, researchers can now assess single enantiomers of existing or new PET radiotracers and evaluate if these compounds offer any benefits over the enantiomeric combinations. Eventually, the goal is to use this chemistry to identify a completely new PET radiotracer for imaging.
Currently, there are only four US Food and Drug Administration (FDA)-approved 18F radiotracers. One of the major limitations to discovering PET tracers is the fact that the only commercially available source of 18F is nucleophilic fluoride. Existing 18F sources are very basic, and during the process of making the 18F radiotracer, can cause the elimination of alcohol and amine groups and rearrange the groups around a chiral center in a process called racemization. Under Dr. Doyle’s less basic reaction conditions, even alcohols and secondary amines are tolerated and no racemization is seen.
“Forming carbon-fluorine bonds by nucleophilic fluoride is challenging. One typically needs to use high temperatures or else the reactions are too slow to permit radioisotope incorporation,” Dr. Doyle commented. “Whereas most reactions require temperatures greater than 100° Celsius, our reaction can be run at 50° Celsius.”
Small amounts of radioactivity were sufficient to develop the reaction at first but to perform imaging studies, larger amounts of radioactivity are necessary. “When you go to higher activity, that’s when you do automated chemistry in a hot cell, which is basically a block of lead so you get no exposure,” Dr. Doyle said.
To be efficient in an industrial environment, the chemistry needs to be converted from the laboratory to an automated hot cell. The researchers were given access to an automated hot cell nearby at Merck’s West Point (PA, USA) site. The whole process of radiolabeling takes about 30 to 45 minutes when it is automated. The set-up includes a robotic arm that delivers solutions to designated vials, a high-performance liquid chromatography (HPLC) system and a rotary evaporator, which are devices for the analysis and purification of the radiotracers.
“The catalyst is very robust and the fact that we can translate the reaction directly to the hot cell bodes very well for non-experts to be able to run these sorts of reactions,” Dr. Doyle concluded. “We demonstrated that the radioactivity is high enough that we could actually use it for imaging. That’s an exciting next step.”
Related Links:
Princeton University
Latest Nuclear Medicine News
- Radiopharmaceutical Molecule Marker to Improve Choice of Bladder Cancer Therapies
- Cancer “Flashlight” Shows Who Can Benefit from Targeted Treatments
- PET Imaging of Inflammation Predicts Recovery and Guides Therapy After Heart Attack
- Radiotheranostic Approach Detects, Kills and Reprograms Aggressive Cancers
- New Imaging Solution Improves Survival for Patients with Recurring Prostate Cancer
- PET Tracer Enables Same-Day Imaging of Triple-Negative Breast and Urothelial Cancers
- New Camera Sees Inside Human Body for Enhanced Scanning and Diagnosis
- Novel Bacteria-Specific PET Imaging Approach Detects Hard-To-Diagnose Lung Infections
- New Imaging Approach Could Reduce Need for Biopsies to Monitor Prostate Cancer
- Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
- Novel PET Imaging Approach Offers Never-Before-Seen View of Neuroinflammation
- Novel Radiotracer Identifies Biomarker for Triple-Negative Breast Cancer
- Innovative PET Imaging Technique to Help Diagnose Neurodegeneration
- New Molecular Imaging Test to Improve Lung Cancer Diagnosis
- Novel PET Technique Visualizes Spinal Cord Injuries to Predict Recovery
- Next-Gen Tau Radiotracers Outperform FDA-Approved Imaging Agents in Detecting Alzheimer’s
Channels
Radiography
view channel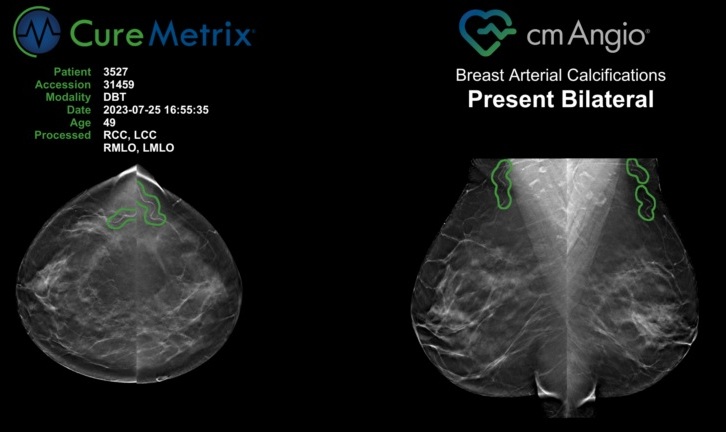
Routine Mammograms Could Predict Future Cardiovascular Disease in Women
Mammograms are widely used to screen for breast cancer, but they may also contain overlooked clues about cardiovascular health. Calcium deposits in the arteries of the breast signal stiffening blood vessels,... Read more
AI Detects Early Signs of Aging from Chest X-Rays
Chronological age does not always reflect how fast the body is truly aging, and current biological age tests often rely on DNA-based markers that may miss early organ-level decline. Detecting subtle, age-related... Read moreMRI
view channel
MRI Scans Reveal Signature Patterns of Brain Activity to Predict Recovery from TBI
Recovery after traumatic brain injury (TBI) varies widely, with some patients regaining full function while others are left with lasting disabilities. Prognosis is especially difficult to assess in patients... Read more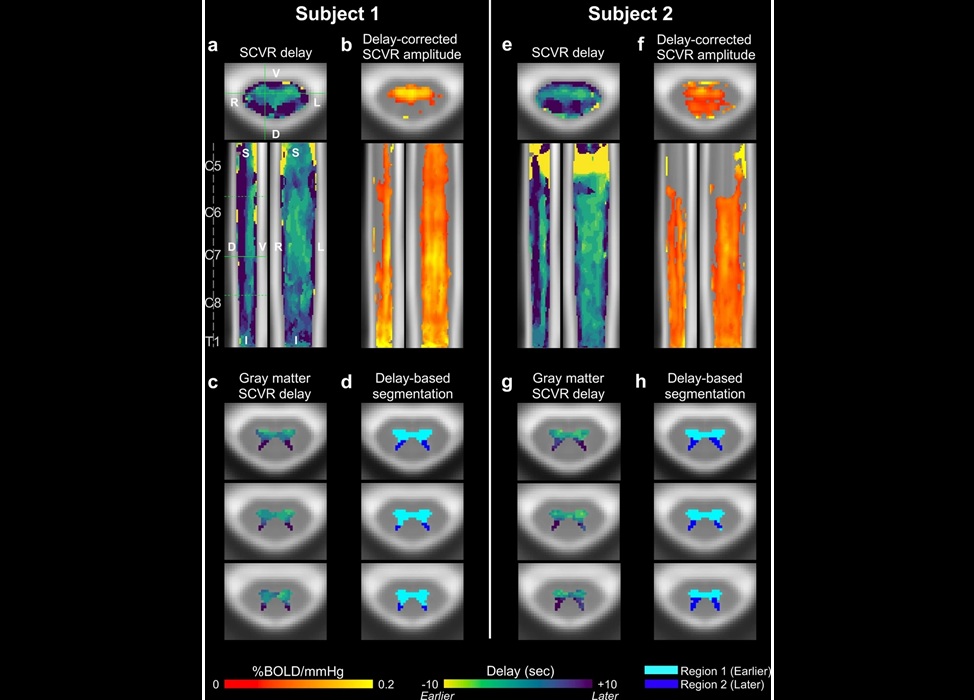
Novel Imaging Approach to Improve Treatment for Spinal Cord Injuries
Vascular dysfunction in the spinal cord contributes to multiple neurological conditions, including traumatic injuries and degenerative cervical myelopathy, where reduced blood flow can lead to progressive... Read more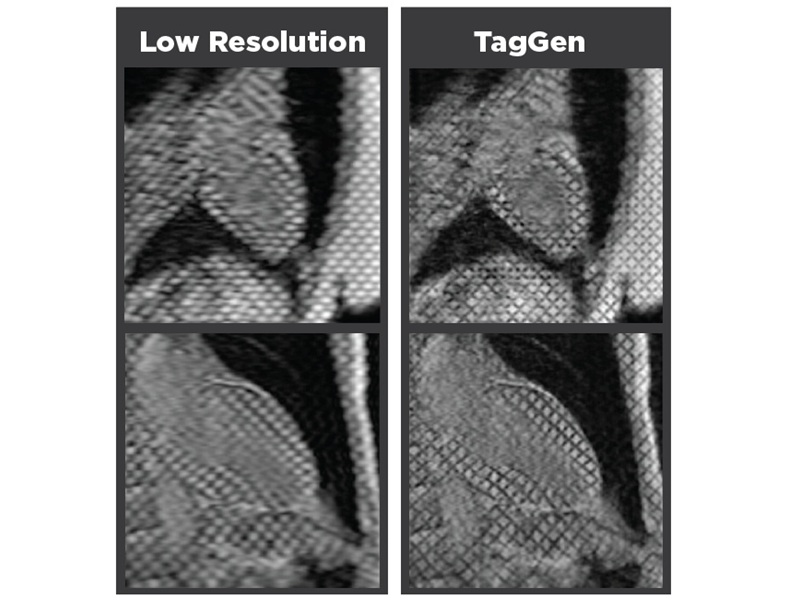
AI-Assisted Model Enhances MRI Heart Scans
A cardiac MRI can reveal critical information about the heart’s function and any abnormalities, but traditional scans take 30 to 90 minutes and often suffer from poor image quality due to patient movement.... Read more
AI Model Outperforms Doctors at Identifying Patients Most At-Risk of Cardiac Arrest
Hypertrophic cardiomyopathy is one of the most common inherited heart conditions and a leading cause of sudden cardiac death in young individuals and athletes. While many patients live normal lives, some... Read moreUltrasound
view channel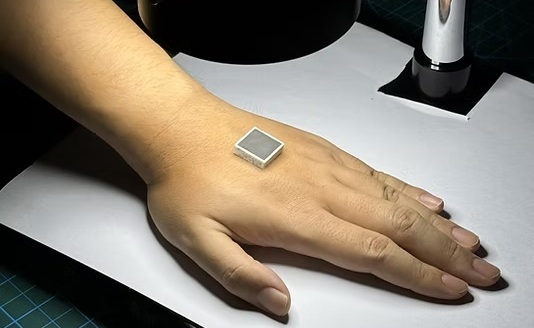
Wearable Ultrasound Imaging System to Enable Real-Time Disease Monitoring
Chronic conditions such as hypertension and heart failure require close monitoring, yet today’s ultrasound imaging is largely confined to hospitals and short, episodic scans. This reactive model limits... Read more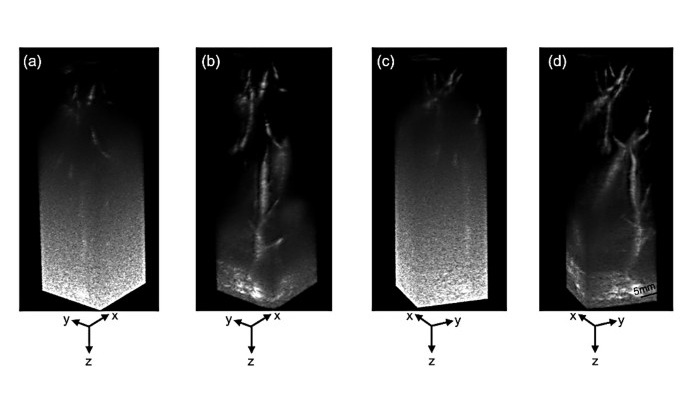
Ultrasound Technique Visualizes Deep Blood Vessels in 3D Without Contrast Agents
Producing clear 3D images of deep blood vessels has long been difficult without relying on contrast agents, CT scans, or MRI. Standard ultrasound typically provides only 2D cross-sections, limiting clinicians’... Read moreGeneral/Advanced Imaging
view channel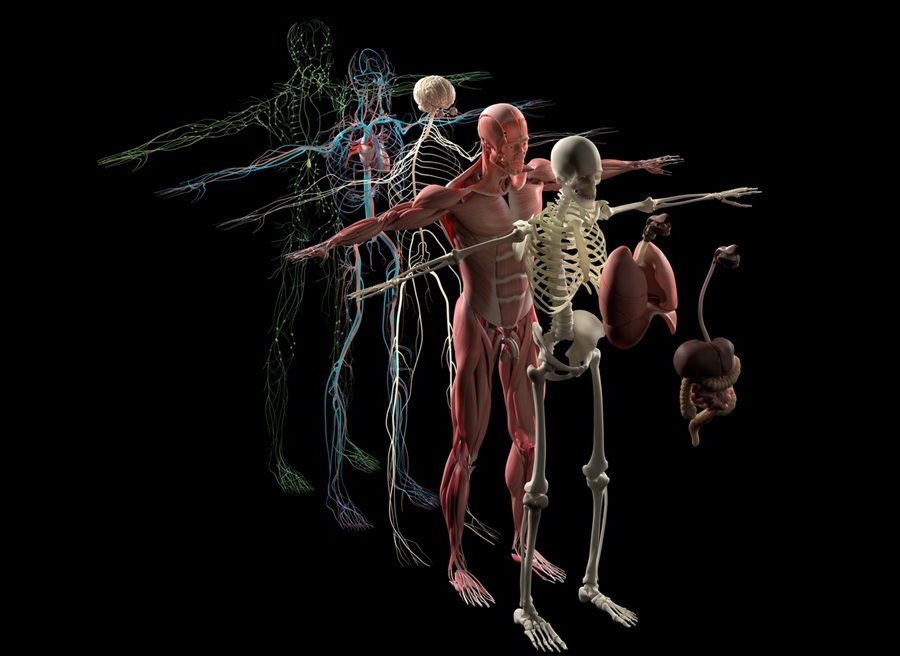
New 3D Imaging System Addresses MRI, CT and Ultrasound Limitations
Medical imaging is central to diagnosing and managing injuries, cancer, infections, and chronic diseases, yet existing tools each come with trade-offs. Ultrasound, X-ray, CT, and MRI can be costly, time-consuming,... Read more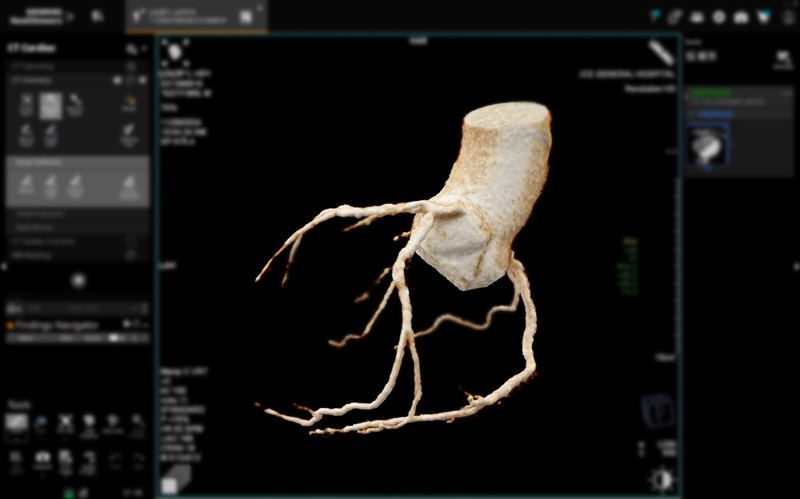
AI-Based Tool Predicts Future Cardiovascular Events in Angina Patients
Stable coronary artery disease is a common cause of chest pain, yet accurately identifying patients at the highest risk of future heart attacks or death remains difficult. Standard coronary CT scans show... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel
GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
GE HealthCare (Chicago, IL, USA) has entered into a collaboration with NVIDIA (Santa Clara, CA, USA), expanding the existing relationship between the two companies to focus on pioneering innovation in... Read more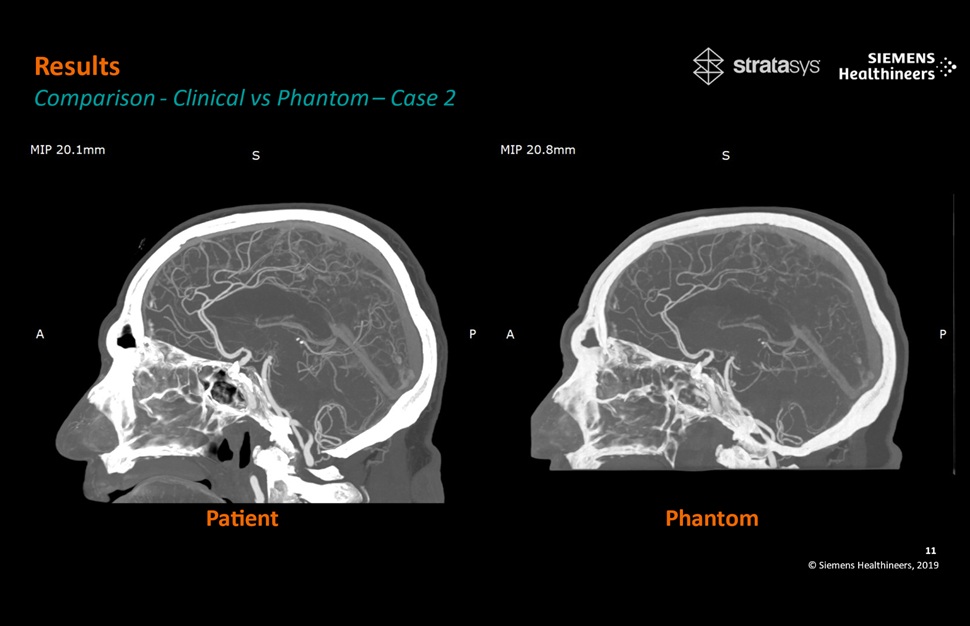
Patient-Specific 3D-Printed Phantoms Transform CT Imaging
New research has highlighted how anatomically precise, patient-specific 3D-printed phantoms are proving to be scalable, cost-effective, and efficient tools in the development of new CT scan algorithms... Read more
Siemens and Sectra Collaborate on Enhancing Radiology Workflows
Siemens Healthineers (Forchheim, Germany) and Sectra (Linköping, Sweden) have entered into a collaboration aimed at enhancing radiologists' diagnostic capabilities and, in turn, improving patient care... Read more