Philips and Glygenix Therapeutics to Develop Ultrasound-Mediated Gene Therapy
|
By MedImaging International staff writers Posted on 21 Aug 2009 |
Philips Healthcare (Best, The Netherlands) and GlyGenix Therapeutics, Inc. (Woodbridge, CT, USA) announced a joint research agreement to research the feasibility of using ultrasound technologies for gene therapy. In particular, the collaboration will explore the treatment of glycogen storage disease-type 1a (GSD-1a) in preclinical studies.
The collaboration unites Philips' expertise in medical imaging technologies for diagnosis and minimally invasive medical procedures with GlyGenix's expertise in correcting the genetic defect in GSD-1a. "The potential to deliver genes using a targeted approach will be a significant advance for correcting genetic defects and could offer the prospect of curing hereditary diseases such as GSD-1a,” commented William Fodor, CSO of GlyGenix Therapeutics. "Philips' ultrasound-mediated DNA delivery techniques offer the opportunity to deliver genes without the size constraints and limitations of viral packaging systems, and thus open the door to the development of more robust and effective therapeutic genes.”
"Medical imaging systems already play a crucial role in minimally-invasive medical procedures such as opening obstructed arteries, correcting heart rhythm disorders, or sampling tissue biopsies of suspected lesions,” said Henk van Houten, senior vice president of Philips Research and head of the healthcare research program. "The development of ultrasound techniques that could noninvasively target the delivery of drugs, genes, and stem cells to specific parts of the body opens up further possibilities to advance patient care.”
GSD-1a is an inherited disease that makes it impossible for the body to regulate blood sugar (glucose) levels, due to a defective G6Pase gene that prevents the body from producing an enzyme called glucose-6-phosphatase. Although it is a rare disease, only affecting approximately 1 in every 100,000-200,000 births in the United States alone, it results in a significant reduction in patients' quality of life and can lead to potentially life-threatening comorbidities in early adulthood. Currently, there are no approved curative treatments for GSD-1a. Correcting the genetic defect that causes it could offer the prospect of an effective therapy that would allow patients with GSD-1a to lead a normal life.
Current gene therapies that rely solely on the bloodstream to deliver corrective gene molecules typically fail to deliver sufficient quantities to the target organs. However, by directing focused ultrasound to target organs following DNA delivery, an increase in uptake via a process known as sonoporation has been successfully demonstrated in pre-clinical studies. Sonoporation increases the permeability of cell walls to allow the uptake of large molecules, thereby enabling the delivery of therapeutic genes.
Compared to current gene therapies that use viral vectors to infect cells, this ultrasound-mediated technique carries no risk of an antiviral immune or inflammatory response. In addition, this targeted approach could reduce side effects.
The proposed treatment is known as ultrasound-mediated plasmid DNA (pDNA) delivery. The research program into it will specifically target the expression of a functional human G6Pase therapeutic pDNA to the liver, the primary organ responsible for glycogen storage and glucose release. Preclinical studies to investigate the feasibility of the technique will be performed by Philips Research and GlyGenix Therapeutics in collaboration with the Duke University School of Medicine's division of medical genetics (Durham, NC, USA), a recognized leader in GSD-1a diagnosis, managed care, pediatric genetics, and experimental models.
GlyGenix Therapeutics holds a worldwide exclusive license to the G6Pase gene, protein, and related mutations for the treatment of GSD-1a. GlyGenix will seek to obtain orphan drug designation for the treatment of GSD-1a, which would provide seven years of market exclusivity.
GlyGenix Therapeutics is a privately held biotech company developing therapeutic solutions for severe metabolic disorders. The company's initial focus is in using gene therapy products with nonviral delivery systems for the treatment of GSD1a, a rare and severe chronic genetic liver disease for which no approved therapies exist. With the aid of a sizable body of preclinical data, GlyGenix Therapeutics, Inc is poised to expedite the initiation of clinical trials for the treatment of GSD1a.
Related Links:
Philips Healthcare
GlyGenix Therapeutics
The collaboration unites Philips' expertise in medical imaging technologies for diagnosis and minimally invasive medical procedures with GlyGenix's expertise in correcting the genetic defect in GSD-1a. "The potential to deliver genes using a targeted approach will be a significant advance for correcting genetic defects and could offer the prospect of curing hereditary diseases such as GSD-1a,” commented William Fodor, CSO of GlyGenix Therapeutics. "Philips' ultrasound-mediated DNA delivery techniques offer the opportunity to deliver genes without the size constraints and limitations of viral packaging systems, and thus open the door to the development of more robust and effective therapeutic genes.”
"Medical imaging systems already play a crucial role in minimally-invasive medical procedures such as opening obstructed arteries, correcting heart rhythm disorders, or sampling tissue biopsies of suspected lesions,” said Henk van Houten, senior vice president of Philips Research and head of the healthcare research program. "The development of ultrasound techniques that could noninvasively target the delivery of drugs, genes, and stem cells to specific parts of the body opens up further possibilities to advance patient care.”
GSD-1a is an inherited disease that makes it impossible for the body to regulate blood sugar (glucose) levels, due to a defective G6Pase gene that prevents the body from producing an enzyme called glucose-6-phosphatase. Although it is a rare disease, only affecting approximately 1 in every 100,000-200,000 births in the United States alone, it results in a significant reduction in patients' quality of life and can lead to potentially life-threatening comorbidities in early adulthood. Currently, there are no approved curative treatments for GSD-1a. Correcting the genetic defect that causes it could offer the prospect of an effective therapy that would allow patients with GSD-1a to lead a normal life.
Current gene therapies that rely solely on the bloodstream to deliver corrective gene molecules typically fail to deliver sufficient quantities to the target organs. However, by directing focused ultrasound to target organs following DNA delivery, an increase in uptake via a process known as sonoporation has been successfully demonstrated in pre-clinical studies. Sonoporation increases the permeability of cell walls to allow the uptake of large molecules, thereby enabling the delivery of therapeutic genes.
Compared to current gene therapies that use viral vectors to infect cells, this ultrasound-mediated technique carries no risk of an antiviral immune or inflammatory response. In addition, this targeted approach could reduce side effects.
The proposed treatment is known as ultrasound-mediated plasmid DNA (pDNA) delivery. The research program into it will specifically target the expression of a functional human G6Pase therapeutic pDNA to the liver, the primary organ responsible for glycogen storage and glucose release. Preclinical studies to investigate the feasibility of the technique will be performed by Philips Research and GlyGenix Therapeutics in collaboration with the Duke University School of Medicine's division of medical genetics (Durham, NC, USA), a recognized leader in GSD-1a diagnosis, managed care, pediatric genetics, and experimental models.
GlyGenix Therapeutics holds a worldwide exclusive license to the G6Pase gene, protein, and related mutations for the treatment of GSD-1a. GlyGenix will seek to obtain orphan drug designation for the treatment of GSD-1a, which would provide seven years of market exclusivity.
GlyGenix Therapeutics is a privately held biotech company developing therapeutic solutions for severe metabolic disorders. The company's initial focus is in using gene therapy products with nonviral delivery systems for the treatment of GSD1a, a rare and severe chronic genetic liver disease for which no approved therapies exist. With the aid of a sizable body of preclinical data, GlyGenix Therapeutics, Inc is poised to expedite the initiation of clinical trials for the treatment of GSD1a.
Related Links:
Philips Healthcare
GlyGenix Therapeutics
Latest Industry News News
- GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
- Patient-Specific 3D-Printed Phantoms Transform CT Imaging
- Siemens and Sectra Collaborate on Enhancing Radiology Workflows
- Bracco Diagnostics and ColoWatch Partner to Expand Availability CRC Screening Tests Using Virtual Colonoscopy
- Mindray Partners with TeleRay to Streamline Ultrasound Delivery
- Philips and Medtronic Partner on Stroke Care
- Siemens and Medtronic Enter into Global Partnership for Advancing Spine Care Imaging Technologies
- RSNA 2024 Technical Exhibits to Showcase Latest Advances in Radiology
- Bracco Collaborates with Arrayus on Microbubble-Assisted Focused Ultrasound Therapy for Pancreatic Cancer
- Innovative Collaboration to Enhance Ischemic Stroke Detection and Elevate Standards in Diagnostic Imaging
- RSNA 2024 Registration Opens
- Microsoft collaborates with Leading Academic Medical Systems to Advance AI in Medical Imaging
- GE HealthCare Acquires Intelligent Ultrasound Group’s Clinical Artificial Intelligence Business
- Bayer and Rad AI Collaborate on Expanding Use of Cutting Edge AI Radiology Operational Solutions
- Polish Med-Tech Company BrainScan to Expand Extensively into Foreign Markets
- Hologic Acquires UK-Based Breast Surgical Guidance Company Endomagnetics Ltd.
Channels
Radiography
view channel
AI Radiology Tool Identifies Life-Threatening Conditions in Milliseconds
Radiology is emerging as one of healthcare’s most pressing bottlenecks. By 2033, the U.S. could face a shortage of up to 42,000 radiologists, even as imaging volumes grow by 5% annually.... Read more
Machine Learning Algorithm Identifies Cardiovascular Risk from Routine Bone Density Scans
A new study published in the Journal of Bone and Mineral Research reveals that an automated machine learning program can predict the risk of cardiovascular events and falls or fractures by analyzing bone... Read more
AI Improves Early Detection of Interval Breast Cancers
Interval breast cancers, which occur between routine screenings, are easier to treat when detected earlier. Early detection can reduce the need for aggressive treatments and improve the chances of better outcomes.... Read more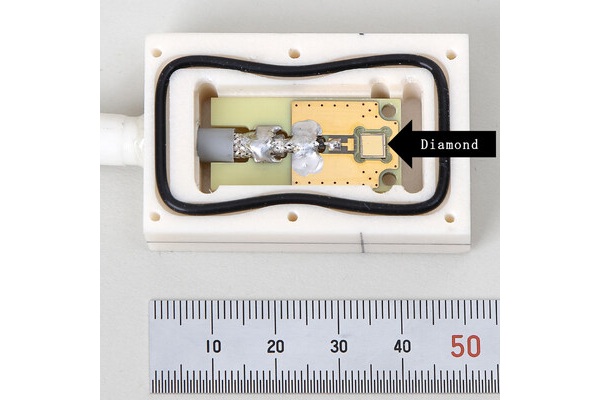
World's Largest Class Single Crystal Diamond Radiation Detector Opens New Possibilities for Diagnostic Imaging
Diamonds possess ideal physical properties for radiation detection, such as exceptional thermal and chemical stability along with a quick response time. Made of carbon with an atomic number of six, diamonds... Read moreMRI
view channel
New MRI Technique Reveals Hidden Heart Issues
Traditional exercise stress tests conducted within an MRI machine require patients to lie flat, a position that artificially improves heart function by increasing stroke volume due to gravity-driven blood... Read more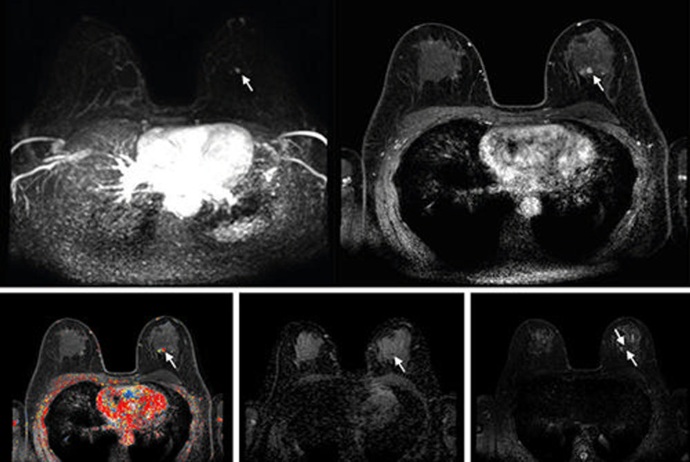
Shorter MRI Exam Effectively Detects Cancer in Dense Breasts
Women with extremely dense breasts face a higher risk of missed breast cancer diagnoses, as dense glandular and fibrous tissue can obscure tumors on mammograms. While breast MRI is recommended for supplemental... Read moreUltrasound
view channel
New Medical Ultrasound Imaging Technique Enables ICU Bedside Monitoring
Ultrasound computed tomography (USCT) presents a safer alternative to imaging techniques like X-ray computed tomography (commonly known as CT or “CAT” scans) because it does not produce ionizing radiation.... Read more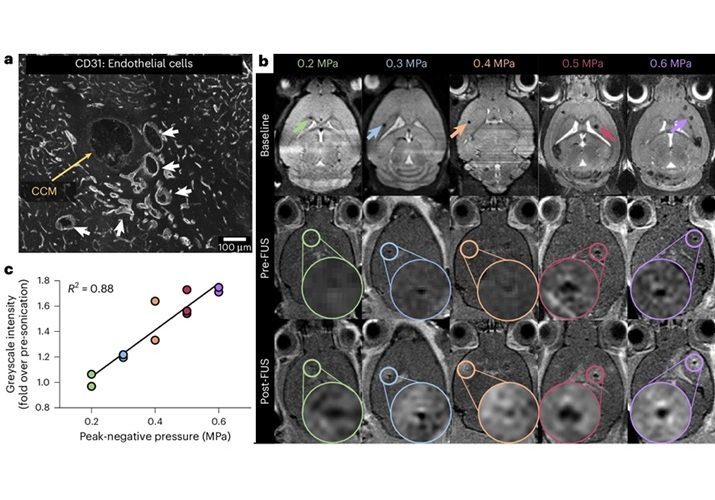
New Incision-Free Technique Halts Growth of Debilitating Brain Lesions
Cerebral cavernous malformations (CCMs), also known as cavernomas, are abnormal clusters of blood vessels that can grow in the brain, spinal cord, or other parts of the body. While most cases remain asymptomatic,... Read moreNuclear Medicine
view channel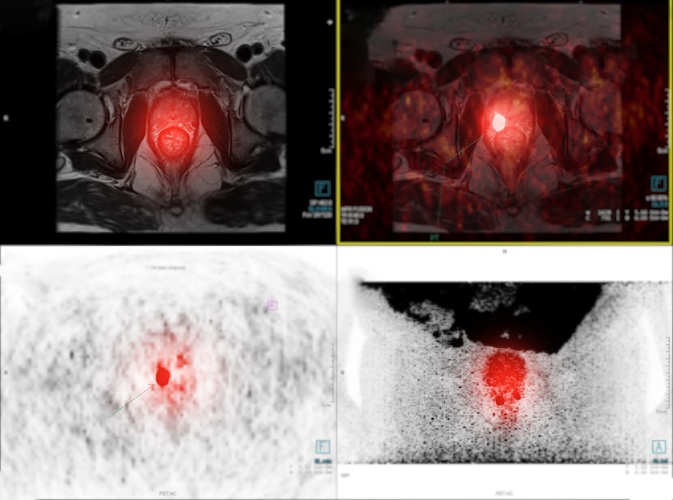
New Imaging Approach Could Reduce Need for Biopsies to Monitor Prostate Cancer
Prostate cancer is the second leading cause of cancer-related death among men in the United States. However, the majority of older men diagnosed with prostate cancer have slow-growing, low-risk forms of... Read more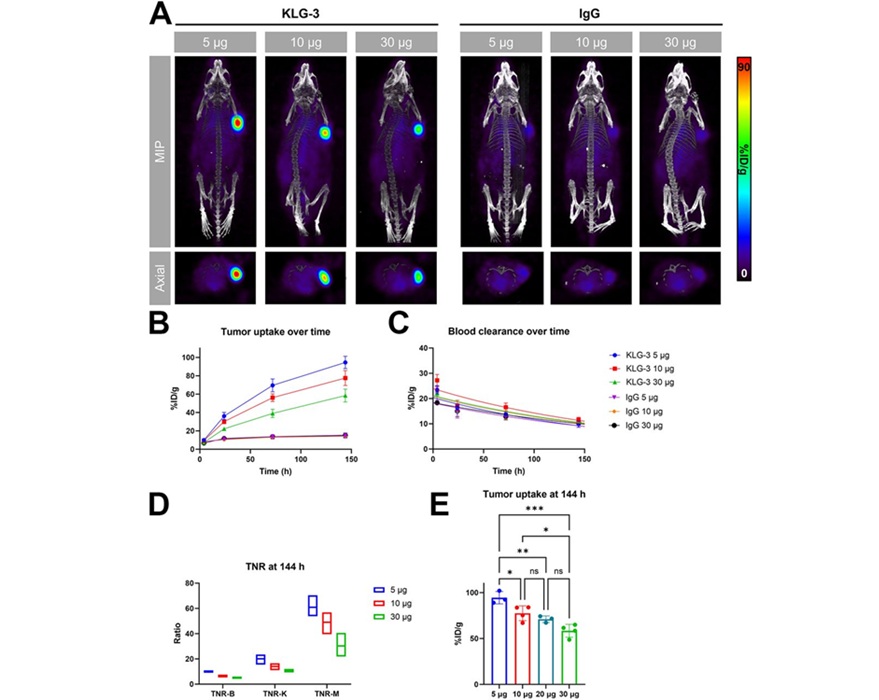
Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
Interleukin-13 receptor α-2 (IL13Rα2) is a cell surface receptor commonly found in solid tumors such as glioblastoma, melanoma, and breast cancer. It is minimally expressed in normal tissues, making it... Read moreGeneral/Advanced Imaging
view channel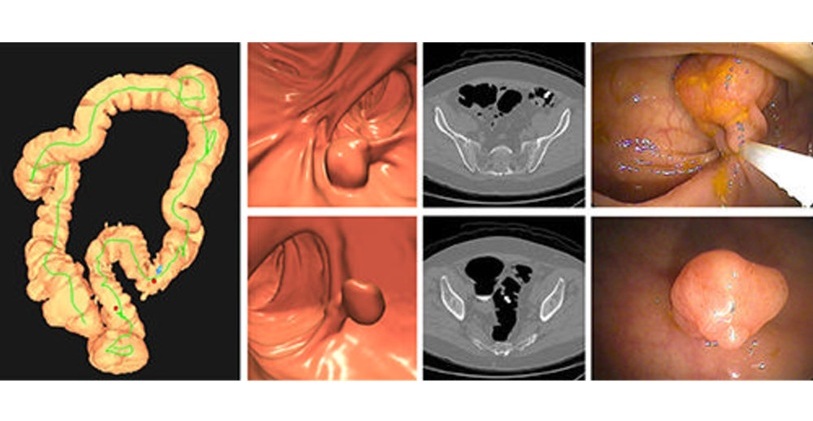
CT Colonography Beats Stool DNA Testing for Colon Cancer Screening
As colorectal cancer remains the second leading cause of cancer-related deaths worldwide, early detection through screening is vital to reduce advanced-stage treatments and associated costs.... Read more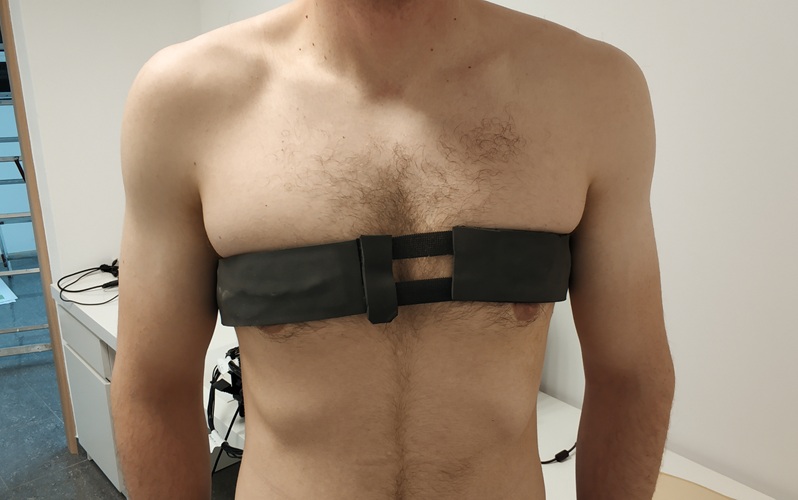
First-Of-Its-Kind Wearable Device Offers Revolutionary Alternative to CT Scans
Currently, patients with conditions such as heart failure, pneumonia, or respiratory distress often require multiple imaging procedures that are intermittent, disruptive, and involve high levels of radiation.... Read more
AI-Based CT Scan Analysis Predicts Early-Stage Kidney Damage Due to Cancer Treatments
Radioligand therapy, a form of targeted nuclear medicine, has recently gained attention for its potential in treating specific types of tumors. However, one of the potential side effects of this therapy... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more












.jpeg)