US Report Outlines Nanotech Scientific, Regulatory Challenges
|
By MedImaging staff writers Posted on 09 Aug 2007 |
The U.S. Food and Drug Administration (FDA; Rockville, MD, USA) Nanotechnology Task Force recently released a report that recommends the agency consider developing guidance and taking other steps to address the benefits and risks of drugs and medical devices using nanotechnology.
"Nanotechnology holds enormous potential for use in a vast array of products,” said Commissioner of Food and Drugs Andrew von Eschenbach, M.D., who endorsed the Task Force Report and its recommendations on July 23, 2007. "Recognizing the emerging nature of this technology and its potential for rapid development, this report fosters the continued development of innovative, safe, and effective FDA-regulated products that use nanotechnology materials.”
Scientists and researchers increasingly are working in the nanoscale, creating and using materials and devices at the level of molecules and atoms--1/100,000th the width of a human hair.
The FDA's Task Force Report on Nanotechnology addresses regulatory and scientific issues and recommends the FDA consider development of nanotechnology-associated guidance for manufacturers and researchers. The Task Force was initiated by Commissioner von Eschenbach in 2006.
The Task Force reported that nanoscale materials potentially could be used in most product types regulated by FDA, and that those materials present challenges similar to those posed by products using other emerging technologies. The challenges, however, may be complicated by the fact that properties pertinent to product safety and effectiveness may change as size varies within the nanoscale.
The report also stated that the emerging and uncertain nature of nanotechnology and the potentially rapid development of applications for FDA-regulated products underscore the need for ensuring transparent, consistent, and predictable regulatory pathways.
Foreseeing the potential for rapid development in the field, the report recommends consideration of agency guidance that would clarify, for example, what information to give FDA about products, and also when the use of nanoscale materials may alter the regulatory status of particular products. As with other FDA guidance, draft guidance documents would be made available for public comment prior to being finalized.
Furthermore, the report says the FDA should work to evaluate data needs to better regulate nanotechnology products, including biologic effects and interactions of nanoscale substances. The agency also should develop in-house expertise and ensure consideration of relevant new information on nanotechnology as it becomes available, according to the report. The FDA should evaluate the efficacy of current testing approaches to assess safety, effectiveness, and quality of nanoscale materials.
The FDA and 22 other federal agencies are part of the U.S. National Nanotechnology Initiative, a federal research and development program established to coordinate the multi-agency efforts in nanoscale science, engineering, and technology.
Related Links:
U.S. Food and Drug Administration
"Nanotechnology holds enormous potential for use in a vast array of products,” said Commissioner of Food and Drugs Andrew von Eschenbach, M.D., who endorsed the Task Force Report and its recommendations on July 23, 2007. "Recognizing the emerging nature of this technology and its potential for rapid development, this report fosters the continued development of innovative, safe, and effective FDA-regulated products that use nanotechnology materials.”
Scientists and researchers increasingly are working in the nanoscale, creating and using materials and devices at the level of molecules and atoms--1/100,000th the width of a human hair.
The FDA's Task Force Report on Nanotechnology addresses regulatory and scientific issues and recommends the FDA consider development of nanotechnology-associated guidance for manufacturers and researchers. The Task Force was initiated by Commissioner von Eschenbach in 2006.
The Task Force reported that nanoscale materials potentially could be used in most product types regulated by FDA, and that those materials present challenges similar to those posed by products using other emerging technologies. The challenges, however, may be complicated by the fact that properties pertinent to product safety and effectiveness may change as size varies within the nanoscale.
The report also stated that the emerging and uncertain nature of nanotechnology and the potentially rapid development of applications for FDA-regulated products underscore the need for ensuring transparent, consistent, and predictable regulatory pathways.
Foreseeing the potential for rapid development in the field, the report recommends consideration of agency guidance that would clarify, for example, what information to give FDA about products, and also when the use of nanoscale materials may alter the regulatory status of particular products. As with other FDA guidance, draft guidance documents would be made available for public comment prior to being finalized.
Furthermore, the report says the FDA should work to evaluate data needs to better regulate nanotechnology products, including biologic effects and interactions of nanoscale substances. The agency also should develop in-house expertise and ensure consideration of relevant new information on nanotechnology as it becomes available, according to the report. The FDA should evaluate the efficacy of current testing approaches to assess safety, effectiveness, and quality of nanoscale materials.
The FDA and 22 other federal agencies are part of the U.S. National Nanotechnology Initiative, a federal research and development program established to coordinate the multi-agency efforts in nanoscale science, engineering, and technology.
Related Links:
U.S. Food and Drug Administration
Latest Industry News News
- GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
- Patient-Specific 3D-Printed Phantoms Transform CT Imaging
- Siemens and Sectra Collaborate on Enhancing Radiology Workflows
- Bracco Diagnostics and ColoWatch Partner to Expand Availability CRC Screening Tests Using Virtual Colonoscopy
- Mindray Partners with TeleRay to Streamline Ultrasound Delivery
- Philips and Medtronic Partner on Stroke Care
- Siemens and Medtronic Enter into Global Partnership for Advancing Spine Care Imaging Technologies
- RSNA 2024 Technical Exhibits to Showcase Latest Advances in Radiology
- Bracco Collaborates with Arrayus on Microbubble-Assisted Focused Ultrasound Therapy for Pancreatic Cancer
- Innovative Collaboration to Enhance Ischemic Stroke Detection and Elevate Standards in Diagnostic Imaging
- RSNA 2024 Registration Opens
- Microsoft collaborates with Leading Academic Medical Systems to Advance AI in Medical Imaging
- GE HealthCare Acquires Intelligent Ultrasound Group’s Clinical Artificial Intelligence Business
- Bayer and Rad AI Collaborate on Expanding Use of Cutting Edge AI Radiology Operational Solutions
- Polish Med-Tech Company BrainScan to Expand Extensively into Foreign Markets
- Hologic Acquires UK-Based Breast Surgical Guidance Company Endomagnetics Ltd.
Channels
Radiography
view channel
Machine Learning Algorithm Identifies Cardiovascular Risk from Routine Bone Density Scans
A new study published in the Journal of Bone and Mineral Research reveals that an automated machine learning program can predict the risk of cardiovascular events and falls or fractures by analyzing bone... Read more
AI Improves Early Detection of Interval Breast Cancers
Interval breast cancers, which occur between routine screenings, are easier to treat when detected earlier. Early detection can reduce the need for aggressive treatments and improve the chances of better outcomes.... Read more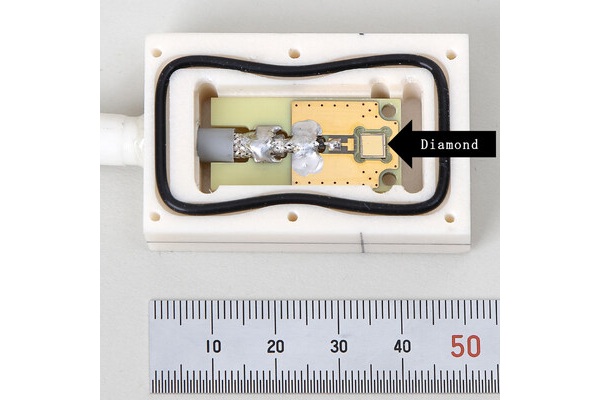
World's Largest Class Single Crystal Diamond Radiation Detector Opens New Possibilities for Diagnostic Imaging
Diamonds possess ideal physical properties for radiation detection, such as exceptional thermal and chemical stability along with a quick response time. Made of carbon with an atomic number of six, diamonds... Read moreMRI
view channel
Simple Brain Scan Diagnoses Parkinson's Disease Years Before It Becomes Untreatable
Parkinson's disease (PD) remains a challenging condition to treat, with no known cure. Though therapies have improved over time, and ongoing research focuses on methods to slow or alter the disease’s progression,... Read more
Cutting-Edge MRI Technology to Revolutionize Diagnosis of Common Heart Problem
Aortic stenosis is a common and potentially life-threatening heart condition. It occurs when the aortic valve, which regulates blood flow from the heart to the rest of the body, becomes stiff and narrow.... Read moreUltrasound
view channel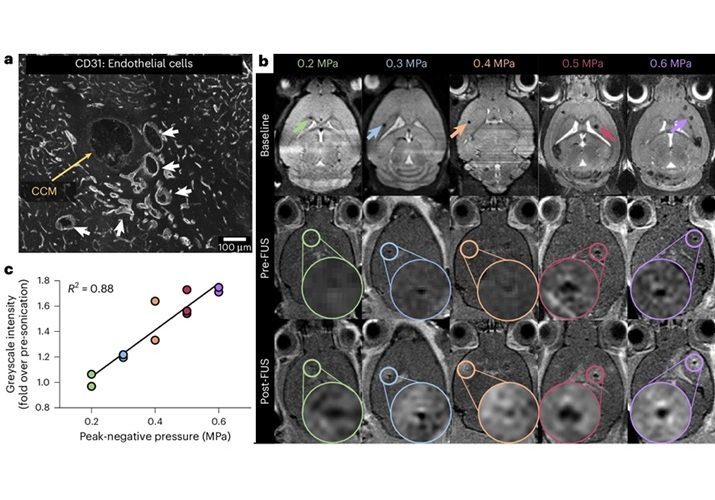
New Incision-Free Technique Halts Growth of Debilitating Brain Lesions
Cerebral cavernous malformations (CCMs), also known as cavernomas, are abnormal clusters of blood vessels that can grow in the brain, spinal cord, or other parts of the body. While most cases remain asymptomatic,... Read more.jpeg)
AI-Powered Lung Ultrasound Outperforms Human Experts in Tuberculosis Diagnosis
Despite global declines in tuberculosis (TB) rates in previous years, the incidence of TB rose by 4.6% from 2020 to 2023. Early screening and rapid diagnosis are essential elements of the World Health... Read moreNuclear Medicine
view channel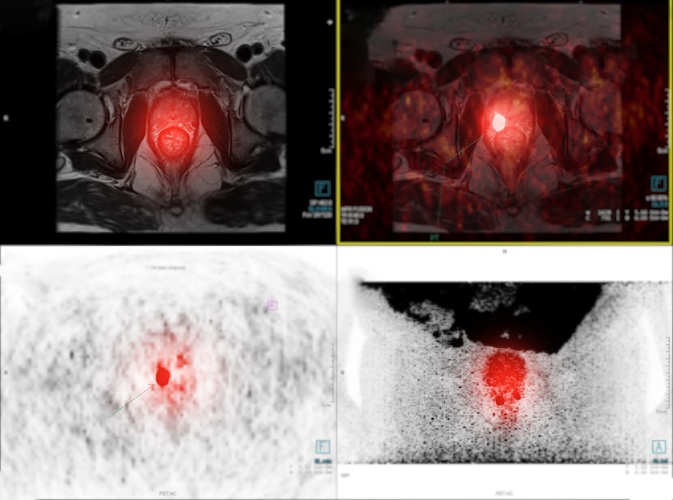
New Imaging Approach Could Reduce Need for Biopsies to Monitor Prostate Cancer
Prostate cancer is the second leading cause of cancer-related death among men in the United States. However, the majority of older men diagnosed with prostate cancer have slow-growing, low-risk forms of... Read more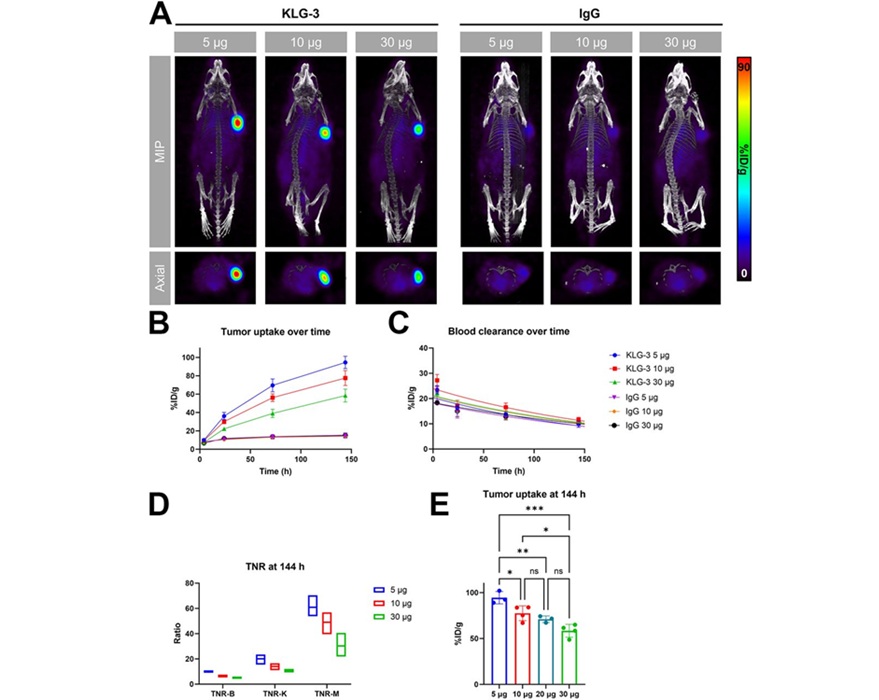
Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
Interleukin-13 receptor α-2 (IL13Rα2) is a cell surface receptor commonly found in solid tumors such as glioblastoma, melanoma, and breast cancer. It is minimally expressed in normal tissues, making it... Read moreGeneral/Advanced Imaging
view channel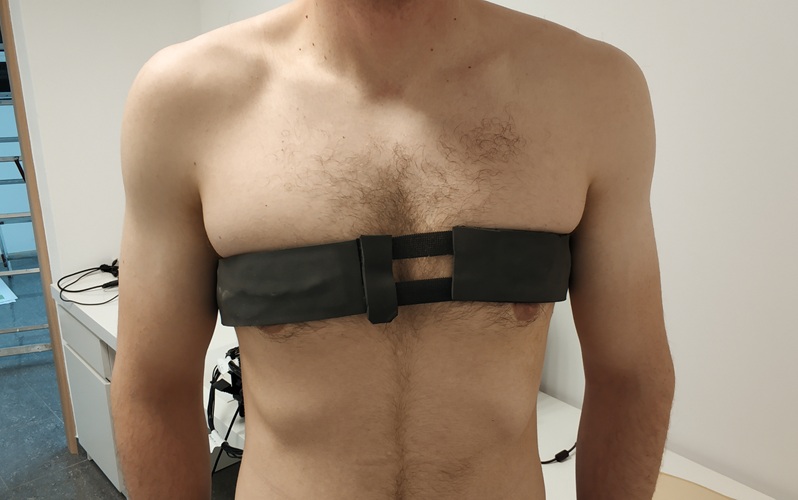
First-Of-Its-Kind Wearable Device Offers Revolutionary Alternative to CT Scans
Currently, patients with conditions such as heart failure, pneumonia, or respiratory distress often require multiple imaging procedures that are intermittent, disruptive, and involve high levels of radiation.... Read more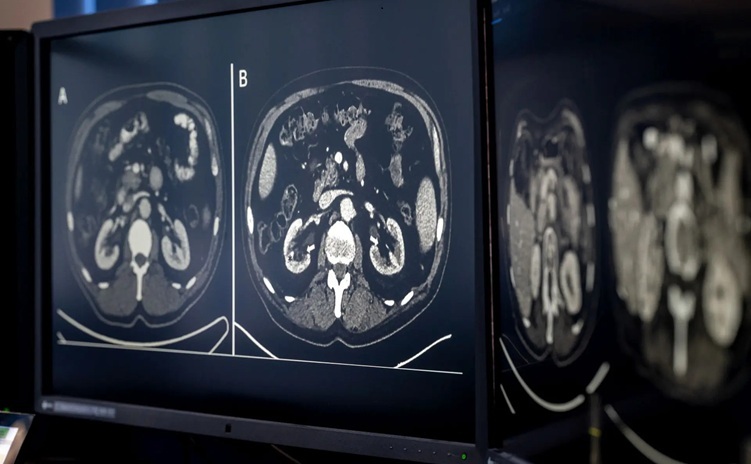
AI-Based CT Scan Analysis Predicts Early-Stage Kidney Damage Due to Cancer Treatments
Radioligand therapy, a form of targeted nuclear medicine, has recently gained attention for its potential in treating specific types of tumors. However, one of the potential side effects of this therapy... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more










 Guided Devices.jpg)