FDA Approval for New Cardiac Devices in Advanced Diagnostic Imaging
|
By MedImaging International staff writers Posted on 26 Oct 2016 |
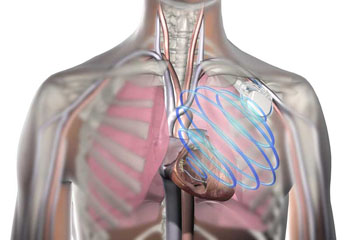
Image: The OptiVol fluid status monitoring for monitor heart failure in patients with a Medtronic ICD (Photo courtesy of Medtronic).
The US Food and Drug Administration (FDA) has approved a suite of cardiac rhythm and heart failure devices, including leads, for use in 3T and 1.5T Magnetic Resonance Imaging (MRI) machines.
The FDA approval will allow patients fitted with MR-conditional pacemakers, Cardiac Resynchronization Therapy-Defibrillators (CRT-Ds), Implantable Cardioverter-Defibrillators (ICDs), and their leads, to undergo MRI scans.
The approved devices are the Medtronic (Dublin, Ireland) Advisa MRI, and Micra Transcatheter Pacemakers, Amplia MRI and Compia MRI CRT-Ds, Evera MRI and Visia AF MRI DF-1 and DF4 ICDs, Reveal LINQ insertable cardiac monitor, SureScan pacing, defibrillation and left-heart leads. Medtronic is a large medical technology, services and solutions company and manufactures a range of devices for interventional, and surgical treatment of cardiac arrhythmias, and cardiovascular disease.
Yair Safriel, MD, neuroradiologist, CMO, Pharmascan Clinical Trials, and University of South Florida, said, "While 1.5T scanners still comprise the majority of installations, 3T scanners are expected to comprise more than half of new units - with some centers having only 3T scanners - since they offer faster scans and higher resolution images. Approval for MRI conditional scanning at both 1.5 and 3T allows patients to have improved access to MRI at a time and place most appropriate for their care. And with 3T scanning, physicians and radiologists gain a clearer look into soft tissues, particularly critical when diagnosing serious conditions, often involving the brain and spine."
Related Links:
Medtronic
The FDA approval will allow patients fitted with MR-conditional pacemakers, Cardiac Resynchronization Therapy-Defibrillators (CRT-Ds), Implantable Cardioverter-Defibrillators (ICDs), and their leads, to undergo MRI scans.
The approved devices are the Medtronic (Dublin, Ireland) Advisa MRI, and Micra Transcatheter Pacemakers, Amplia MRI and Compia MRI CRT-Ds, Evera MRI and Visia AF MRI DF-1 and DF4 ICDs, Reveal LINQ insertable cardiac monitor, SureScan pacing, defibrillation and left-heart leads. Medtronic is a large medical technology, services and solutions company and manufactures a range of devices for interventional, and surgical treatment of cardiac arrhythmias, and cardiovascular disease.
Yair Safriel, MD, neuroradiologist, CMO, Pharmascan Clinical Trials, and University of South Florida, said, "While 1.5T scanners still comprise the majority of installations, 3T scanners are expected to comprise more than half of new units - with some centers having only 3T scanners - since they offer faster scans and higher resolution images. Approval for MRI conditional scanning at both 1.5 and 3T allows patients to have improved access to MRI at a time and place most appropriate for their care. And with 3T scanning, physicians and radiologists gain a clearer look into soft tissues, particularly critical when diagnosing serious conditions, often involving the brain and spine."
Related Links:
Medtronic
Latest MRI News
- World's First Sensor Detects Errors in MRI Scans Using Laser Light and Gas
- Diamond Dust Could Offer New Contrast Agent Option for Future MRI Scans
- Combining MRI with PSA Testing Improves Clinical Outcomes for Prostate Cancer Patients
- PET/MRI Improves Diagnostic Accuracy for Prostate Cancer Patients
- Next Generation MR-Guided Focused Ultrasound Ushers In Future of Incisionless Neurosurgery
- Two-Part MRI Scan Detects Prostate Cancer More Quickly without Compromising Diagnostic Quality
- World’s Most Powerful MRI Machine Images Living Brain with Unrivaled Clarity
- New Whole-Body Imaging Technology Makes It Possible to View Inflammation on MRI Scan
- Combining Prostate MRI with Blood Test Can Avoid Unnecessary Prostate Biopsies
- New Treatment Combines MRI and Ultrasound to Control Prostate Cancer without Serious Side Effects
- MRI Improves Diagnosis and Treatment of Prostate Cancer
- Combined PET-MRI Scan Improves Treatment for Early Breast Cancer Patients
- 4D MRI Could Improve Clinical Assessment of Heart Blood Flow Abnormalities
- MRI-Guided Focused Ultrasound Therapy Shows Promise in Treating Prostate Cancer
- AI-Based MRI Tool Outperforms Current Brain Tumor Diagnosis Methods
- DW-MRI Lights up Small Ovarian Lesions like Light Bulbs
Channels
Radiography
view channel
Novel Breast Imaging System Proves As Effective As Mammography
Breast cancer remains the most frequently diagnosed cancer among women. It is projected that one in eight women will be diagnosed with breast cancer during her lifetime, and one in 42 women who turn 50... Read more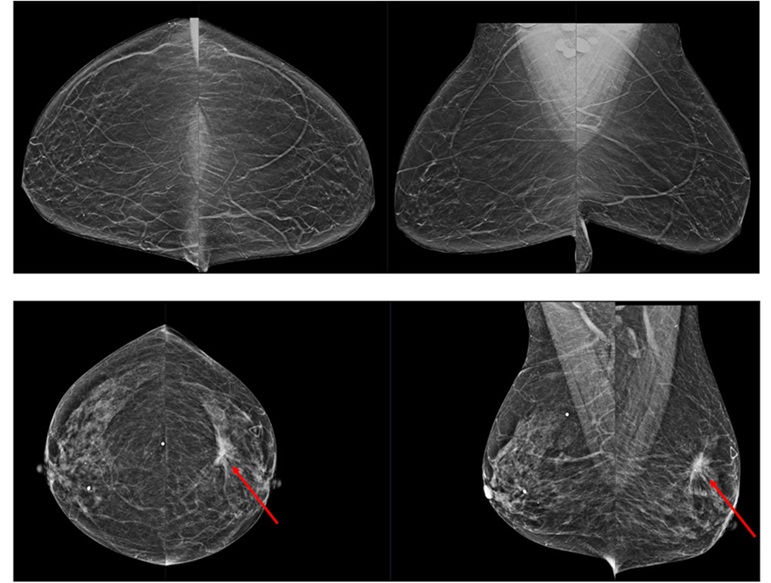
AI Assistance Improves Breast-Cancer Screening by Reducing False Positives
Radiologists typically detect one case of cancer for every 200 mammograms reviewed. However, these evaluations often result in false positives, leading to unnecessary patient recalls for additional testing,... Read moreUltrasound
view channel
Largest Model Trained On Echocardiography Images Assesses Heart Structure and Function
Foundation models represent an exciting frontier in generative artificial intelligence (AI), yet many lack the specialized medical data needed to make them applicable in healthcare settings.... Read more.jpg)
Groundbreaking Technology Enables Precise, Automatic Measurement of Peripheral Blood Vessels
The current standard of care of using angiographic information is often inadequate for accurately assessing vessel size in the estimated 20 million people in the U.S. who suffer from peripheral vascular disease.... Read more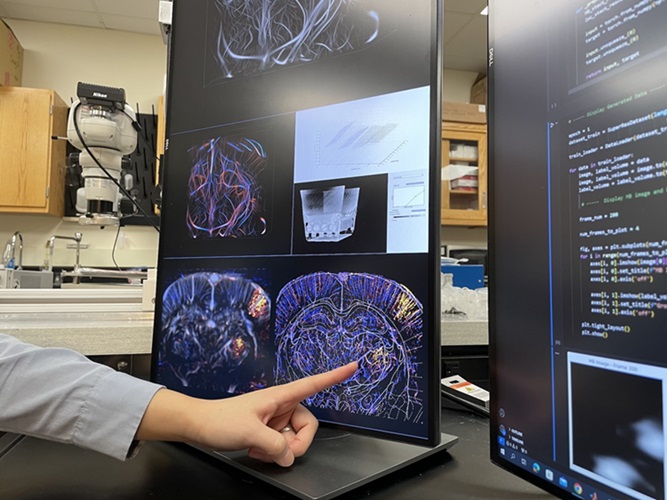
Deep Learning Advances Super-Resolution Ultrasound Imaging
Ultrasound localization microscopy (ULM) is an advanced imaging technique that offers high-resolution visualization of microvascular structures. It employs microbubbles, FDA-approved contrast agents, injected... Read more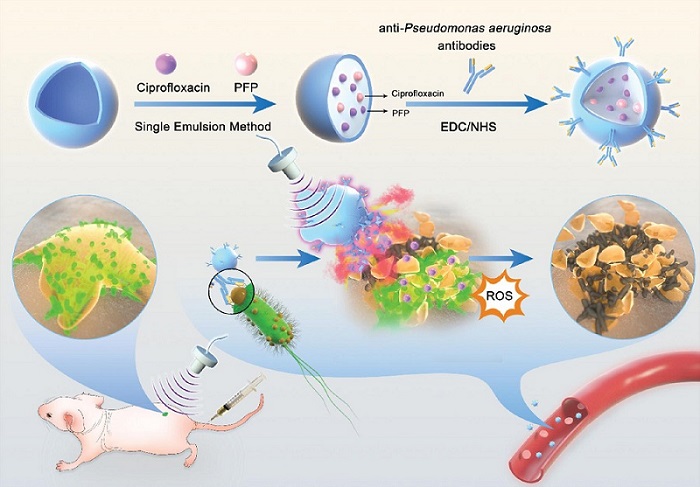
Novel Ultrasound-Launched Targeted Nanoparticle Eliminates Biofilm and Bacterial Infection
Biofilms, formed by bacteria aggregating into dense communities for protection against harsh environmental conditions, are a significant contributor to various infectious diseases. Biofilms frequently... Read moreNuclear Medicine
view channel
New Imaging Technique Monitors Inflammation Disorders without Radiation Exposure
Imaging inflammation using traditional radiological techniques presents significant challenges, including radiation exposure, poor image quality, high costs, and invasive procedures. Now, new contrast... Read more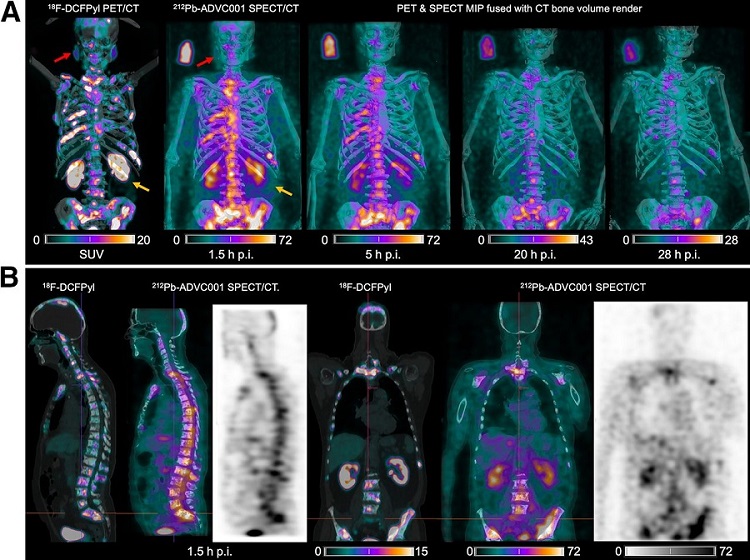
New SPECT/CT Technique Could Change Imaging Practices and Increase Patient Access
The development of lead-212 (212Pb)-PSMA–based targeted alpha therapy (TAT) is garnering significant interest in treating patients with metastatic castration-resistant prostate cancer. The imaging of 212Pb,... Read moreNew Radiotheranostic System Detects and Treats Ovarian Cancer Noninvasively
Ovarian cancer is the most lethal gynecological cancer, with less than a 30% five-year survival rate for those diagnosed in late stages. Despite surgery and platinum-based chemotherapy being the standard... Read more
AI System Automatically and Reliably Detects Cardiac Amyloidosis Using Scintigraphy Imaging
Cardiac amyloidosis, a condition characterized by the buildup of abnormal protein deposits (amyloids) in the heart muscle, severely affects heart function and can lead to heart failure or death without... Read moreGeneral/Advanced Imaging
view channel
PET Scans Reveal Hidden Inflammation in Multiple Sclerosis Patients
A key challenge for clinicians treating patients with multiple sclerosis (MS) is that after a certain amount of time, they continue to worsen even though their MRIs show no change. A new study has now... Read more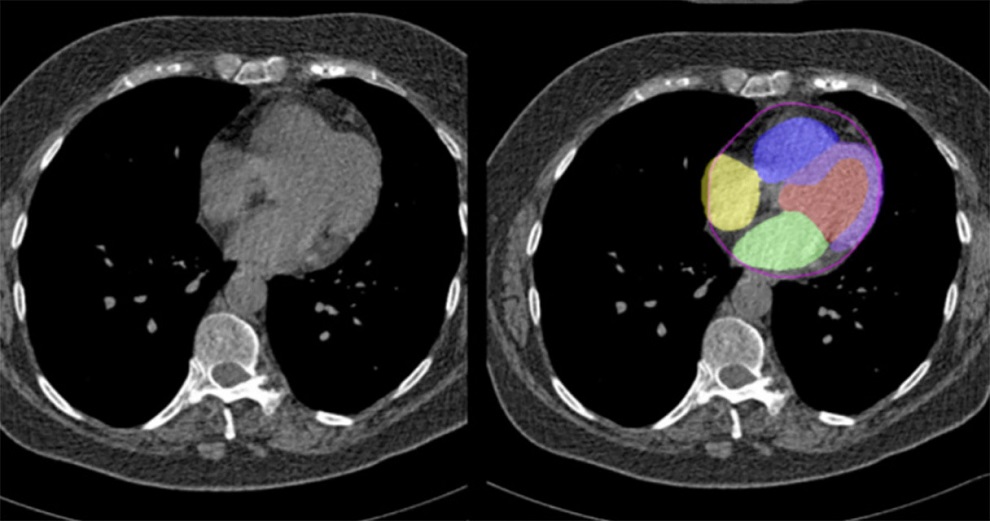
Artificial Intelligence Evaluates Cardiovascular Risk from CT Scans
Chest computed tomography (CT) is a common diagnostic tool, with approximately 15 million scans conducted each year in the United States, though many are underutilized or not fully explored.... Read more
New AI Method Captures Uncertainty in Medical Images
In the field of biomedicine, segmentation is the process of annotating pixels from an important structure in medical images, such as organs or cells. Artificial Intelligence (AI) models are utilized to... Read more.jpg)
CT Coronary Angiography Reduces Need for Invasive Tests to Diagnose Coronary Artery Disease
Coronary artery disease (CAD), one of the leading causes of death worldwide, involves the narrowing of coronary arteries due to atherosclerosis, resulting in insufficient blood flow to the heart muscle.... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel
Bayer and Google Partner on New AI Product for Radiologists
Medical imaging data comprises around 90% of all healthcare data, and it is a highly complex and rich clinical data modality and serves as a vital tool for diagnosing patients. Each year, billions of medical... Read more