FDA Approval for New Cardiac Devices in Advanced Diagnostic Imaging
|
By MedImaging International staff writers Posted on 26 Oct 2016 |
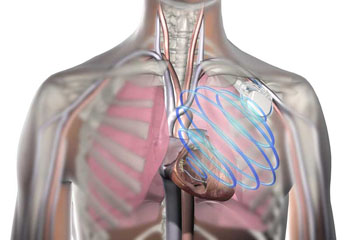
Image: The OptiVol fluid status monitoring for monitor heart failure in patients with a Medtronic ICD (Photo courtesy of Medtronic).
The US Food and Drug Administration (FDA) has approved a suite of cardiac rhythm and heart failure devices, including leads, for use in 3T and 1.5T Magnetic Resonance Imaging (MRI) machines.
The FDA approval will allow patients fitted with MR-conditional pacemakers, Cardiac Resynchronization Therapy-Defibrillators (CRT-Ds), Implantable Cardioverter-Defibrillators (ICDs), and their leads, to undergo MRI scans.
The approved devices are the Medtronic (Dublin, Ireland) Advisa MRI, and Micra Transcatheter Pacemakers, Amplia MRI and Compia MRI CRT-Ds, Evera MRI and Visia AF MRI DF-1 and DF4 ICDs, Reveal LINQ insertable cardiac monitor, SureScan pacing, defibrillation and left-heart leads. Medtronic is a large medical technology, services and solutions company and manufactures a range of devices for interventional, and surgical treatment of cardiac arrhythmias, and cardiovascular disease.
Yair Safriel, MD, neuroradiologist, CMO, Pharmascan Clinical Trials, and University of South Florida, said, "While 1.5T scanners still comprise the majority of installations, 3T scanners are expected to comprise more than half of new units - with some centers having only 3T scanners - since they offer faster scans and higher resolution images. Approval for MRI conditional scanning at both 1.5 and 3T allows patients to have improved access to MRI at a time and place most appropriate for their care. And with 3T scanning, physicians and radiologists gain a clearer look into soft tissues, particularly critical when diagnosing serious conditions, often involving the brain and spine."
Related Links:
Medtronic
The FDA approval will allow patients fitted with MR-conditional pacemakers, Cardiac Resynchronization Therapy-Defibrillators (CRT-Ds), Implantable Cardioverter-Defibrillators (ICDs), and their leads, to undergo MRI scans.
The approved devices are the Medtronic (Dublin, Ireland) Advisa MRI, and Micra Transcatheter Pacemakers, Amplia MRI and Compia MRI CRT-Ds, Evera MRI and Visia AF MRI DF-1 and DF4 ICDs, Reveal LINQ insertable cardiac monitor, SureScan pacing, defibrillation and left-heart leads. Medtronic is a large medical technology, services and solutions company and manufactures a range of devices for interventional, and surgical treatment of cardiac arrhythmias, and cardiovascular disease.
Yair Safriel, MD, neuroradiologist, CMO, Pharmascan Clinical Trials, and University of South Florida, said, "While 1.5T scanners still comprise the majority of installations, 3T scanners are expected to comprise more than half of new units - with some centers having only 3T scanners - since they offer faster scans and higher resolution images. Approval for MRI conditional scanning at both 1.5 and 3T allows patients to have improved access to MRI at a time and place most appropriate for their care. And with 3T scanning, physicians and radiologists gain a clearer look into soft tissues, particularly critical when diagnosing serious conditions, often involving the brain and spine."
Related Links:
Medtronic
Latest MRI News
- AI Tool Tracks Effectiveness of Multiple Sclerosis Treatments Using Brain MRI Scans
- Ultra-Powerful MRI Scans Enable Life-Changing Surgery in Treatment-Resistant Epileptic Patients
- AI-Powered MRI Technology Improves Parkinson’s Diagnoses
- Biparametric MRI Combined with AI Enhances Detection of Clinically Significant Prostate Cancer
- First-Of-Its-Kind AI-Driven Brain Imaging Platform to Better Guide Stroke Treatment Options
- New Model Improves Comparison of MRIs Taken at Different Institutions
- Groundbreaking New Scanner Sees 'Previously Undetectable' Cancer Spread
- First-Of-Its-Kind Tool Analyzes MRI Scans to Measure Brain Aging
- AI-Enhanced MRI Images Make Cancerous Breast Tissue Glow
- AI Model Automatically Segments MRI Images
- New Research Supports Routine Brain MRI Screening in Asymptomatic Late-Stage Breast Cancer Patients
- Revolutionary Portable Device Performs Rapid MRI-Based Stroke Imaging at Patient's Bedside
- AI Predicts After-Effects of Brain Tumor Surgery from MRI Scans
- MRI-First Strategy for Prostate Cancer Detection Proven Safe
- First-Of-Its-Kind 10' x 48' Mobile MRI Scanner Transforms User and Patient Experience
- New Model Makes MRI More Accurate and Reliable
Channels
Radiography
view channel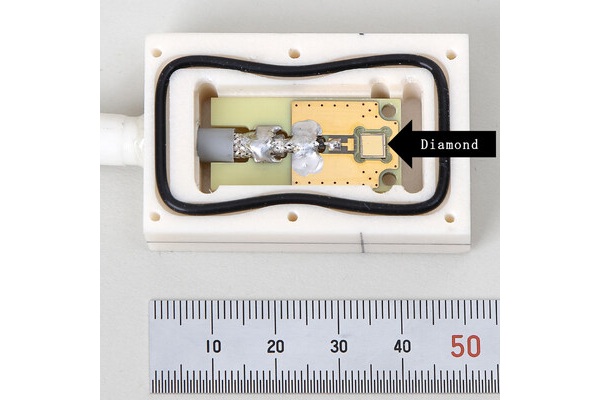
World's Largest Class Single Crystal Diamond Radiation Detector Opens New Possibilities for Diagnostic Imaging
Diamonds possess ideal physical properties for radiation detection, such as exceptional thermal and chemical stability along with a quick response time. Made of carbon with an atomic number of six, diamonds... Read more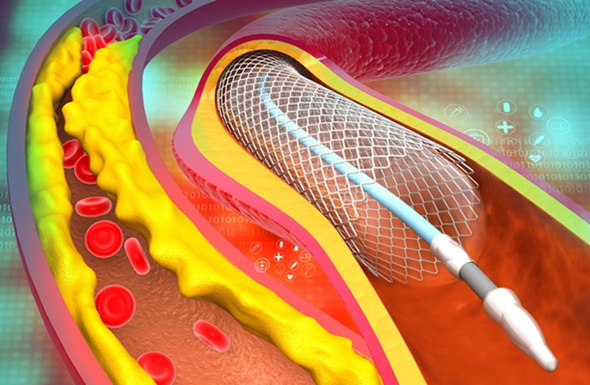
AI-Powered Imaging Technique Shows Promise in Evaluating Patients for PCI
Percutaneous coronary intervention (PCI), also known as coronary angioplasty, is a minimally invasive procedure where small metal tubes called stents are inserted into partially blocked coronary arteries... Read moreUltrasound
view channel.jpeg)
AI-Powered Lung Ultrasound Outperforms Human Experts in Tuberculosis Diagnosis
Despite global declines in tuberculosis (TB) rates in previous years, the incidence of TB rose by 4.6% from 2020 to 2023. Early screening and rapid diagnosis are essential elements of the World Health... Read more
AI Identifies Heart Valve Disease from Common Imaging Test
Tricuspid regurgitation is a condition where the heart's tricuspid valve does not close completely during contraction, leading to backward blood flow, which can result in heart failure. A new artificial... Read moreNuclear Medicine
view channel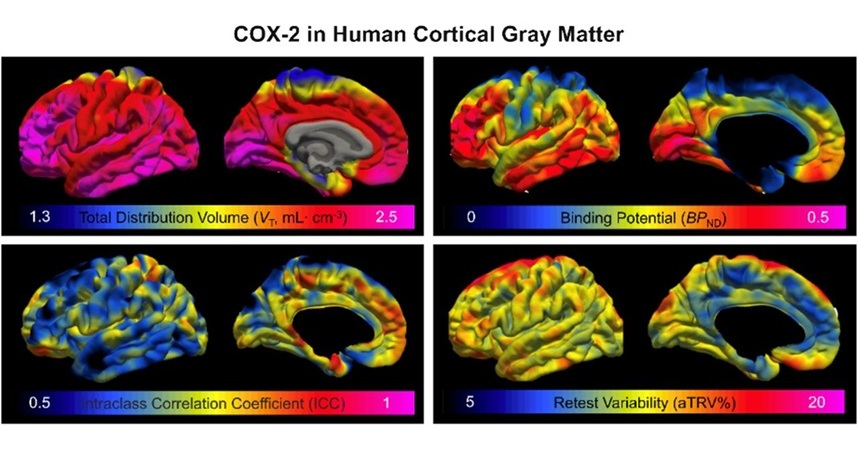
Novel PET Imaging Approach Offers Never-Before-Seen View of Neuroinflammation
COX-2, an enzyme that plays a key role in brain inflammation, can be significantly upregulated by inflammatory stimuli and neuroexcitation. Researchers suggest that COX-2 density in the brain could serve... Read more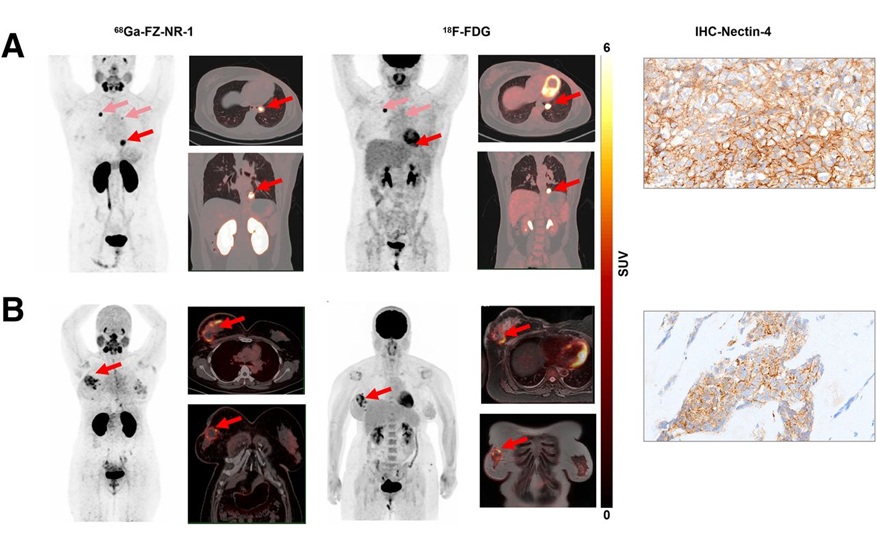
Novel Radiotracer Identifies Biomarker for Triple-Negative Breast Cancer
Triple-negative breast cancer (TNBC), which represents 15-20% of all breast cancer cases, is one of the most aggressive subtypes, with a five-year survival rate of about 40%. Due to its significant heterogeneity... Read moreGeneral/Advanced Imaging
view channel
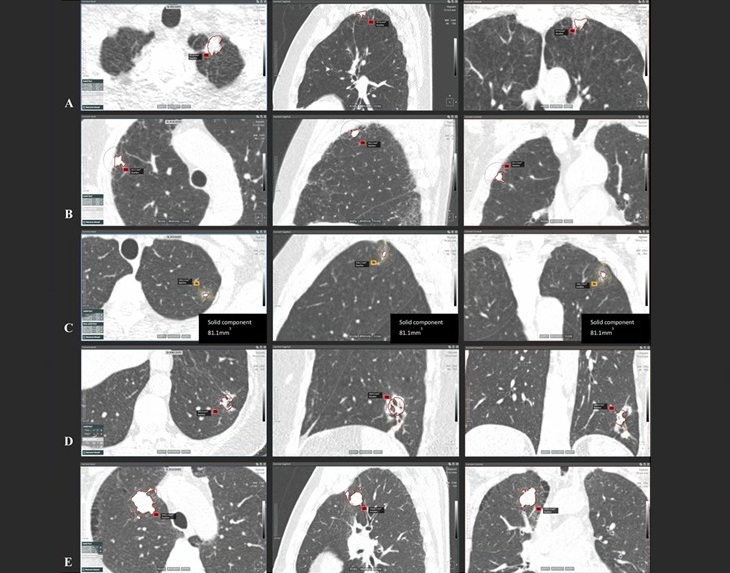
AI-Powered Imaging System Improves Lung Cancer Diagnosis
Given the need to detect lung cancer at earlier stages, there is an increasing need for a definitive diagnostic pathway for patients with suspicious pulmonary nodules. However, obtaining tissue samples... Read more
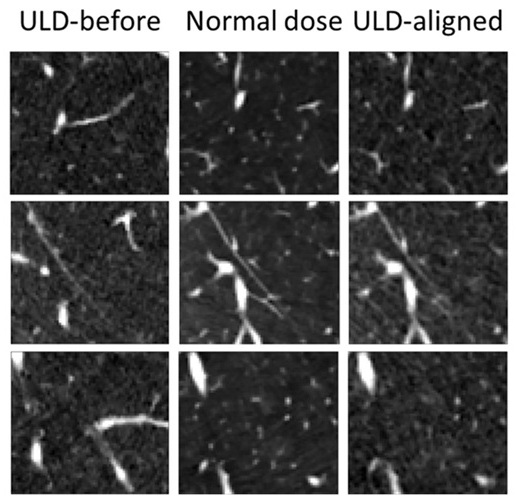
AI Model Significantly Enhances Low-Dose CT Capabilities
Lung cancer remains one of the most challenging diseases, making early diagnosis vital for effective treatment. Fortunately, advancements in artificial intelligence (AI) are revolutionizing lung cancer... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel
GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
GE HealthCare (Chicago, IL, USA) has entered into a collaboration with NVIDIA (Santa Clara, CA, USA), expanding the existing relationship between the two companies to focus on pioneering innovation in... Read more
Patient-Specific 3D-Printed Phantoms Transform CT Imaging
New research has highlighted how anatomically precise, patient-specific 3D-printed phantoms are proving to be scalable, cost-effective, and efficient tools in the development of new CT scan algorithms... Read more
Siemens and Sectra Collaborate on Enhancing Radiology Workflows
Siemens Healthineers (Forchheim, Germany) and Sectra (Linköping, Sweden) have entered into a collaboration aimed at enhancing radiologists' diagnostic capabilities and, in turn, improving patient care... Read more