New Guidelines on MRI Safety and Compatibility of Medical Devices
|
By MedImaging International staff writers Posted on 13 Jan 2015 |

Image: Endovascular grafts, such as W.L. Gore’s TAG thoracic endoprosthesis, are among the “passive” implants subject to the new guidance (Photo courtesy of Gore Medical).
The US Food and Drug Administration issued new guidance recommendations on magnetic resonance imaging (MRI) compatibility—or in some instances, incompatibility—of implants that do not require electricity to service their function, such as intracranial aneurysm clips, cardiovascular stents, endovascular grafts, and transprostatic tissue retractors.
The topic of MRI-safe devices, up to now, has mostly centered on devices such as pacemakers, and more recently cochlear implants, both of which use electronics. Now, however, the FDA (Silver Spring, MD, USA) has three principal concerns about patient safety in regards to these so-called “passive” implants in the MRI setting: the displacement forces and torques on magnetic substances, which can turn devices into deadly projectiles; radiofrequency heating that can burn patients; and the distorting effect of implants upon an MRI scan. The FDA recommends four tests described by standard setting institution, American Society for Testing and Materials (ASTM) International, to tackle these three safety issues, and provides additional details about the way those tests should be performed. For example, the guidance reported that “the testing should encompass the range of sizes of the device you intend to market.”
MRI scans are provided in various magnetic field strengths, measured in Teslas. “Although commercial 1.5 T MR systems are currently the most common, 3 T MR systems are becoming more common. A medical device that is classified as MR Conditional in a 1.5 T scanner may not be safe to scan in an MR system with a higher or lower field strength,” the guidance cautioned.
The implant’s labeling should describe the device as “MR Safe,” “MR Unsafe,” or “MR Conditional,” as defined by ASTM International in standard F2503-13. Lastly, the fourth category, “Safety in MRI Not Evaluated,” may be suitable in some instances, but not for those passive implants that are known to present MRI compatibility issues, are a new device type or contain ferromagnetic materials.
Related Links:
US Food and Drug Administration
The topic of MRI-safe devices, up to now, has mostly centered on devices such as pacemakers, and more recently cochlear implants, both of which use electronics. Now, however, the FDA (Silver Spring, MD, USA) has three principal concerns about patient safety in regards to these so-called “passive” implants in the MRI setting: the displacement forces and torques on magnetic substances, which can turn devices into deadly projectiles; radiofrequency heating that can burn patients; and the distorting effect of implants upon an MRI scan. The FDA recommends four tests described by standard setting institution, American Society for Testing and Materials (ASTM) International, to tackle these three safety issues, and provides additional details about the way those tests should be performed. For example, the guidance reported that “the testing should encompass the range of sizes of the device you intend to market.”
MRI scans are provided in various magnetic field strengths, measured in Teslas. “Although commercial 1.5 T MR systems are currently the most common, 3 T MR systems are becoming more common. A medical device that is classified as MR Conditional in a 1.5 T scanner may not be safe to scan in an MR system with a higher or lower field strength,” the guidance cautioned.
The implant’s labeling should describe the device as “MR Safe,” “MR Unsafe,” or “MR Conditional,” as defined by ASTM International in standard F2503-13. Lastly, the fourth category, “Safety in MRI Not Evaluated,” may be suitable in some instances, but not for those passive implants that are known to present MRI compatibility issues, are a new device type or contain ferromagnetic materials.
Related Links:
US Food and Drug Administration
Latest MRI News
- New MRI Technique Reveals Hidden Heart Issues
- Shorter MRI Exam Effectively Detects Cancer in Dense Breasts
- MRI to Replace Painful Spinal Tap for Faster MS Diagnosis
- MRI Scans Can Identify Cardiovascular Disease Ten Years in Advance
- Simple Brain Scan Diagnoses Parkinson's Disease Years Before It Becomes Untreatable
- Cutting-Edge MRI Technology to Revolutionize Diagnosis of Common Heart Problem
- New MRI Technique Reveals True Heart Age to Prevent Attacks and Strokes
- AI Tool Predicts Relapse of Pediatric Brain Cancer from Brain MRI Scans
- AI Tool Tracks Effectiveness of Multiple Sclerosis Treatments Using Brain MRI Scans
- Ultra-Powerful MRI Scans Enable Life-Changing Surgery in Treatment-Resistant Epileptic Patients
- AI-Powered MRI Technology Improves Parkinson’s Diagnoses
- Biparametric MRI Combined with AI Enhances Detection of Clinically Significant Prostate Cancer
- First-Of-Its-Kind AI-Driven Brain Imaging Platform to Better Guide Stroke Treatment Options
- New Model Improves Comparison of MRIs Taken at Different Institutions
- Groundbreaking New Scanner Sees 'Previously Undetectable' Cancer Spread
- First-Of-Its-Kind Tool Analyzes MRI Scans to Measure Brain Aging
Channels
Radiography
view channel
AI Radiology Tool Identifies Life-Threatening Conditions in Milliseconds
Radiology is emerging as one of healthcare’s most pressing bottlenecks. By 2033, the U.S. could face a shortage of up to 42,000 radiologists, even as imaging volumes grow by 5% annually.... Read more
Machine Learning Algorithm Identifies Cardiovascular Risk from Routine Bone Density Scans
A new study published in the Journal of Bone and Mineral Research reveals that an automated machine learning program can predict the risk of cardiovascular events and falls or fractures by analyzing bone... Read more
AI Improves Early Detection of Interval Breast Cancers
Interval breast cancers, which occur between routine screenings, are easier to treat when detected earlier. Early detection can reduce the need for aggressive treatments and improve the chances of better outcomes.... Read more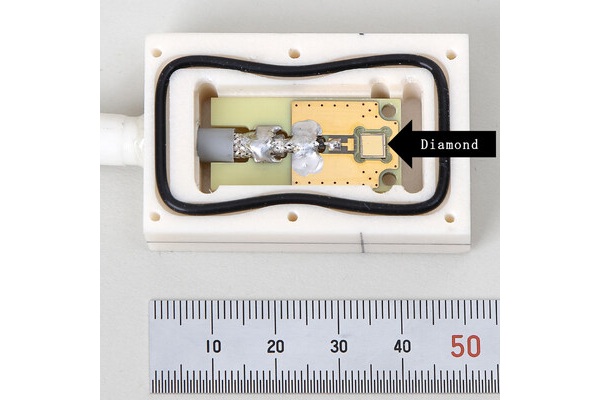
World's Largest Class Single Crystal Diamond Radiation Detector Opens New Possibilities for Diagnostic Imaging
Diamonds possess ideal physical properties for radiation detection, such as exceptional thermal and chemical stability along with a quick response time. Made of carbon with an atomic number of six, diamonds... Read moreUltrasound
view channel
New Medical Ultrasound Imaging Technique Enables ICU Bedside Monitoring
Ultrasound computed tomography (USCT) presents a safer alternative to imaging techniques like X-ray computed tomography (commonly known as CT or “CAT” scans) because it does not produce ionizing radiation.... Read more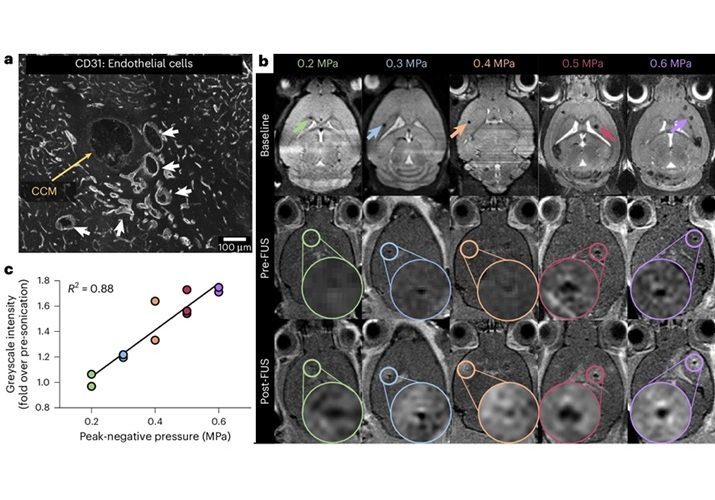
New Incision-Free Technique Halts Growth of Debilitating Brain Lesions
Cerebral cavernous malformations (CCMs), also known as cavernomas, are abnormal clusters of blood vessels that can grow in the brain, spinal cord, or other parts of the body. While most cases remain asymptomatic,... Read moreNuclear Medicine
view channel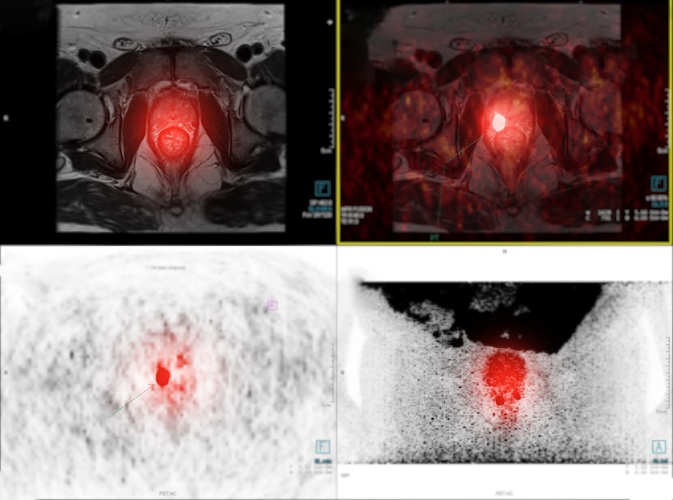
New Imaging Approach Could Reduce Need for Biopsies to Monitor Prostate Cancer
Prostate cancer is the second leading cause of cancer-related death among men in the United States. However, the majority of older men diagnosed with prostate cancer have slow-growing, low-risk forms of... Read more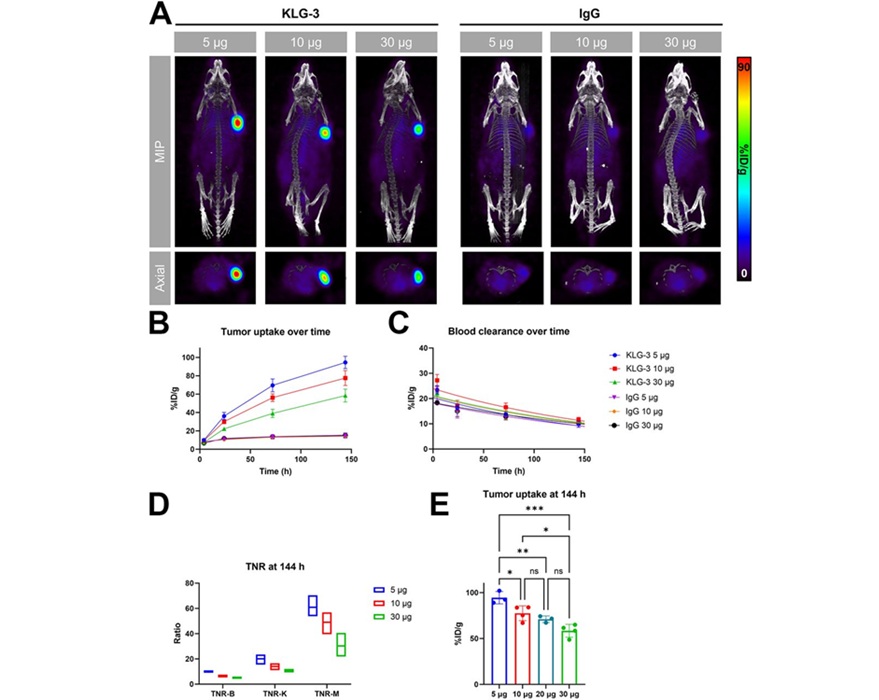
Novel Radiolabeled Antibody Improves Diagnosis and Treatment of Solid Tumors
Interleukin-13 receptor α-2 (IL13Rα2) is a cell surface receptor commonly found in solid tumors such as glioblastoma, melanoma, and breast cancer. It is minimally expressed in normal tissues, making it... Read moreGeneral/Advanced Imaging
view channel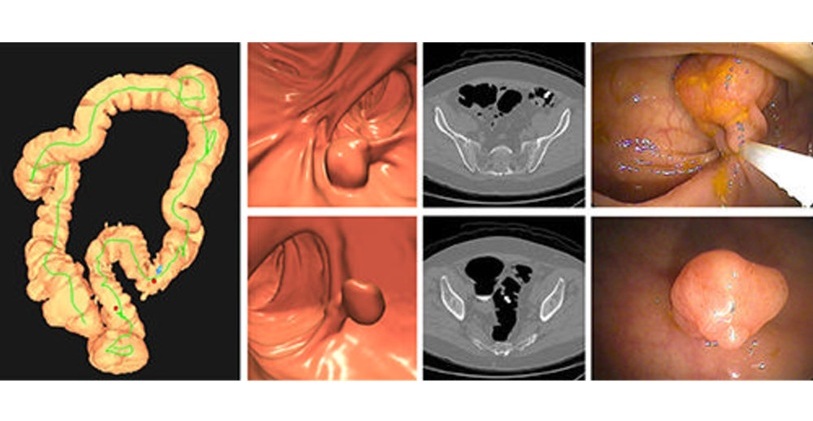
CT Colonography Beats Stool DNA Testing for Colon Cancer Screening
As colorectal cancer remains the second leading cause of cancer-related deaths worldwide, early detection through screening is vital to reduce advanced-stage treatments and associated costs.... Read more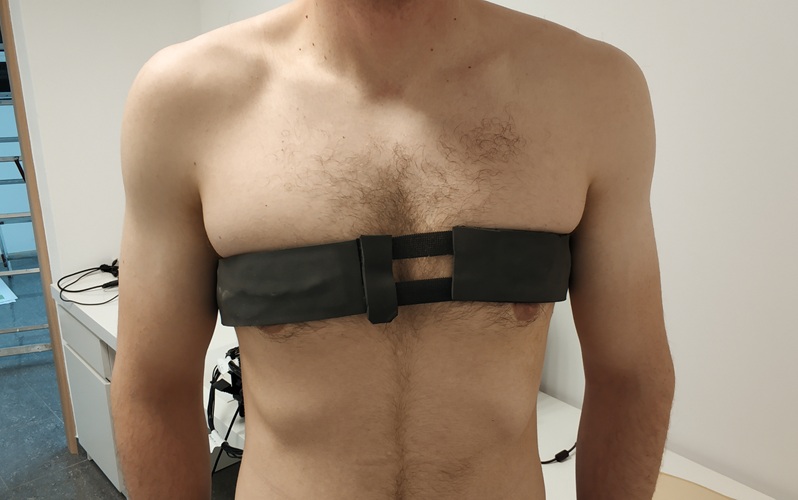
First-Of-Its-Kind Wearable Device Offers Revolutionary Alternative to CT Scans
Currently, patients with conditions such as heart failure, pneumonia, or respiratory distress often require multiple imaging procedures that are intermittent, disruptive, and involve high levels of radiation.... Read more
AI-Based CT Scan Analysis Predicts Early-Stage Kidney Damage Due to Cancer Treatments
Radioligand therapy, a form of targeted nuclear medicine, has recently gained attention for its potential in treating specific types of tumors. However, one of the potential side effects of this therapy... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel
GE HealthCare and NVIDIA Collaboration to Reimagine Diagnostic Imaging
GE HealthCare (Chicago, IL, USA) has entered into a collaboration with NVIDIA (Santa Clara, CA, USA), expanding the existing relationship between the two companies to focus on pioneering innovation in... Read more
Patient-Specific 3D-Printed Phantoms Transform CT Imaging
New research has highlighted how anatomically precise, patient-specific 3D-printed phantoms are proving to be scalable, cost-effective, and efficient tools in the development of new CT scan algorithms... Read more
Siemens and Sectra Collaborate on Enhancing Radiology Workflows
Siemens Healthineers (Forchheim, Germany) and Sectra (Linköping, Sweden) have entered into a collaboration aimed at enhancing radiologists' diagnostic capabilities and, in turn, improving patient care... Read more












.jpeg)