Pacing Lead for Full-Body MRI Scans Approved for Use in Patients with a Slow Heartbeat
|
By MedImaging International staff writers Posted on 21 Oct 2014 |
A new magnetic resonance imaging (MRI) pacing lead is approved in the United States for MRI scans positioned on any region of the body when used with a Medtronic dual-chamber MR-conditional pacemaker for patients with a slow heartbeat.
Medtronic, Inc. (Minneapolis, MN, USA) announced the US Food and Drug Administration (FDA) approval of its CapSureFix Novus MRI SureScan 5076 lead, previously approved for use with Medtronic’s non-MR-conditional pacemakers, has been well-received because of its handling and effectiveness. Since its introduction more than 10 years ago, physicians have implanted more than three million 5076 leads in patients.
With the new FDA approval, two 5076 MRI leads can now be paired with dual-chamber Medtronic Advisa MRI or Revo MRI SureScan pacemakers, allowing patients with these complete SureScan pacing systems to undergo full-body MRI scans. Medtronic 5076 MRI lead lengths approved for these scans range in length from 35–85 cm. Patients who previously had two 5076 leads implanted with non-MRI pacemakers will have the option to receive MRIs if MR-conditional SureScan pacemakers are implanted when replacement devices are needed.
“The 5076 lead has proven to be one of the most reliable pacing leads for more than a decade, and due to extensive testing, now also can undergo MRI scans,” said Brian Urke, vice president and general manager of the bradycardia business at Medtronic. “This is especially important for patients who need MRIs and received 5076 leads at the time of their initial implants; although they may not have received MR-conditional pacemakers initially, they now have the option to get a pacemaker approved for MRI when they require a device change-out, making the entire system MR-conditional.”
Since its first MR-conditional pacing system was approved in Europe in 2008, Medtronic has continued to design and test its products for safe use during MRI scanning. Recent developments in computer modeling have allowed Medtronic to evaluate the 5076 lead across more than two million scanning situations. Furthermore, Medtronic conducted the 5076 MRI Clinical Study, global, multicenter research to evaluate the safety and effectiveness of the 5076 lead in the MRI environment.
Worldwide, it is estimated that up to 75% of patients with implanted cardiac devices are expected to need an MRI scan during the lifetime of their devices. MRI is the standard of care in soft tissue imaging, providing data not seen with X-ray, ultrasound, or computed tomography (CT) scan. MRI is therefore crucial for the early detection and treatment of many diseases. Until recently, patients with implanted pacemakers were refused access to MRI procedures because the interaction can be injurious.
The 5076 MRI lead is the latest addition to a range of Medtronic devices that are approved for MRI access. These include the Medtronic SureScan pacing systems, the SureScan neurostimulation systems for the management of chronic pain, and the SynchroMed II programmable drug infusion system, which are available worldwide. Moreover, the Evera MRI SureScan implantable cardioverter-defibrillator (ICD) system is investigational in the United States.
In collaboration with leading clinicians, researchers, and scientists worldwide, Medtronic offers a wide range of novel medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias.
Related Links:
Medtronic
Medtronic, Inc. (Minneapolis, MN, USA) announced the US Food and Drug Administration (FDA) approval of its CapSureFix Novus MRI SureScan 5076 lead, previously approved for use with Medtronic’s non-MR-conditional pacemakers, has been well-received because of its handling and effectiveness. Since its introduction more than 10 years ago, physicians have implanted more than three million 5076 leads in patients.
With the new FDA approval, two 5076 MRI leads can now be paired with dual-chamber Medtronic Advisa MRI or Revo MRI SureScan pacemakers, allowing patients with these complete SureScan pacing systems to undergo full-body MRI scans. Medtronic 5076 MRI lead lengths approved for these scans range in length from 35–85 cm. Patients who previously had two 5076 leads implanted with non-MRI pacemakers will have the option to receive MRIs if MR-conditional SureScan pacemakers are implanted when replacement devices are needed.
“The 5076 lead has proven to be one of the most reliable pacing leads for more than a decade, and due to extensive testing, now also can undergo MRI scans,” said Brian Urke, vice president and general manager of the bradycardia business at Medtronic. “This is especially important for patients who need MRIs and received 5076 leads at the time of their initial implants; although they may not have received MR-conditional pacemakers initially, they now have the option to get a pacemaker approved for MRI when they require a device change-out, making the entire system MR-conditional.”
Since its first MR-conditional pacing system was approved in Europe in 2008, Medtronic has continued to design and test its products for safe use during MRI scanning. Recent developments in computer modeling have allowed Medtronic to evaluate the 5076 lead across more than two million scanning situations. Furthermore, Medtronic conducted the 5076 MRI Clinical Study, global, multicenter research to evaluate the safety and effectiveness of the 5076 lead in the MRI environment.
Worldwide, it is estimated that up to 75% of patients with implanted cardiac devices are expected to need an MRI scan during the lifetime of their devices. MRI is the standard of care in soft tissue imaging, providing data not seen with X-ray, ultrasound, or computed tomography (CT) scan. MRI is therefore crucial for the early detection and treatment of many diseases. Until recently, patients with implanted pacemakers were refused access to MRI procedures because the interaction can be injurious.
The 5076 MRI lead is the latest addition to a range of Medtronic devices that are approved for MRI access. These include the Medtronic SureScan pacing systems, the SureScan neurostimulation systems for the management of chronic pain, and the SynchroMed II programmable drug infusion system, which are available worldwide. Moreover, the Evera MRI SureScan implantable cardioverter-defibrillator (ICD) system is investigational in the United States.
In collaboration with leading clinicians, researchers, and scientists worldwide, Medtronic offers a wide range of novel medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias.
Related Links:
Medtronic
Latest MRI News
- New Material Boosts MRI Image Quality
- AI Model Reads and Diagnoses Brain MRI in Seconds
- MRI Scan Breakthrough to Help Avoid Risky Invasive Tests for Heart Patients
- MRI Scans Reveal Signature Patterns of Brain Activity to Predict Recovery from TBI
- Novel Imaging Approach to Improve Treatment for Spinal Cord Injuries
- AI-Assisted Model Enhances MRI Heart Scans
- AI Model Outperforms Doctors at Identifying Patients Most At-Risk of Cardiac Arrest
- New MRI Technique Reveals Hidden Heart Issues
- Shorter MRI Exam Effectively Detects Cancer in Dense Breasts
- MRI to Replace Painful Spinal Tap for Faster MS Diagnosis
- MRI Scans Can Identify Cardiovascular Disease Ten Years in Advance
- Simple Brain Scan Diagnoses Parkinson's Disease Years Before It Becomes Untreatable
- Cutting-Edge MRI Technology to Revolutionize Diagnosis of Common Heart Problem
- New MRI Technique Reveals True Heart Age to Prevent Attacks and Strokes
- AI Tool Predicts Relapse of Pediatric Brain Cancer from Brain MRI Scans
- AI Tool Tracks Effectiveness of Multiple Sclerosis Treatments Using Brain MRI Scans
Channels
Radiography
view channel
Routine Mammograms Could Predict Future Cardiovascular Disease in Women
Mammograms are widely used to screen for breast cancer, but they may also contain overlooked clues about cardiovascular health. Calcium deposits in the arteries of the breast signal stiffening blood vessels,... Read more
AI Detects Early Signs of Aging from Chest X-Rays
Chronological age does not always reflect how fast the body is truly aging, and current biological age tests often rely on DNA-based markers that may miss early organ-level decline. Detecting subtle, age-related... Read moreUltrasound
view channel
AI Model Accurately Detects Placenta Accreta in Pregnancy Before Delivery
Placenta accreta spectrum (PAS) is a life-threatening pregnancy complication in which the placenta abnormally attaches to the uterine wall. The condition is a leading cause of maternal mortality and morbidity... Read more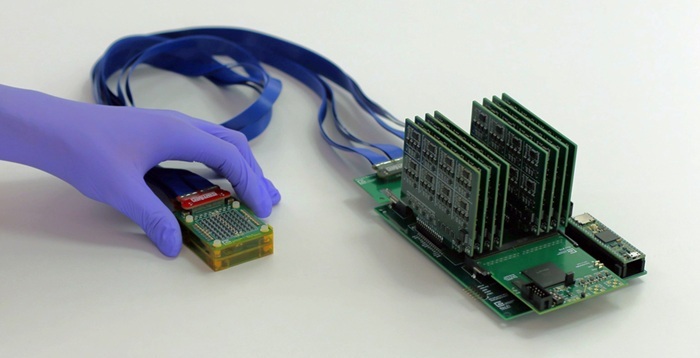
Portable Ultrasound Sensor to Enable Earlier Breast Cancer Detection
Breast cancer screening relies heavily on annual mammograms, but aggressive tumors can develop between scans, accounting for up to 30 percent of cases. These interval cancers are often diagnosed later,... Read more
Portable Imaging Scanner to Diagnose Lymphatic Disease in Real Time
Lymphatic disorders affect hundreds of millions of people worldwide and are linked to conditions ranging from limb swelling and organ dysfunction to birth defects and cancer-related complications.... Read more
Imaging Technique Generates Simultaneous 3D Color Images of Soft-Tissue Structure and Vasculature
Medical imaging tools often force clinicians to choose between speed, structural detail, and functional insight. Ultrasound is fast and affordable but typically limited to two-dimensional anatomy, while... Read moreNuclear Medicine
view channel
Radiopharmaceutical Molecule Marker to Improve Choice of Bladder Cancer Therapies
Targeted cancer therapies only work when tumor cells express the specific molecular structures they are designed to attack. In urothelial carcinoma, a common form of bladder cancer, the cell surface protein... Read more
Cancer “Flashlight” Shows Who Can Benefit from Targeted Treatments
Targeted cancer therapies can be highly effective, but only when a patient’s tumor expresses the specific protein the treatment is designed to attack. Determining this usually requires biopsies or advanced... Read moreGeneral/Advanced Imaging
view channel
AI Tool Offers Prognosis for Patients with Head and Neck Cancer
Oropharyngeal cancer is a form of head and neck cancer that can spread through lymph nodes, significantly affecting survival and treatment decisions. Current therapies often involve combinations of surgery,... Read more
New 3D Imaging System Addresses MRI, CT and Ultrasound Limitations
Medical imaging is central to diagnosing and managing injuries, cancer, infections, and chronic diseases, yet existing tools each come with trade-offs. Ultrasound, X-ray, CT, and MRI can be costly, time-consuming,... Read moreImaging IT
view channel
New Google Cloud Medical Imaging Suite Makes Imaging Healthcare Data More Accessible
Medical imaging is a critical tool used to diagnose patients, and there are billions of medical images scanned globally each year. Imaging data accounts for about 90% of all healthcare data1 and, until... Read more
Global AI in Medical Diagnostics Market to Be Driven by Demand for Image Recognition in Radiology
The global artificial intelligence (AI) in medical diagnostics market is expanding with early disease detection being one of its key applications and image recognition becoming a compelling consumer proposition... Read moreIndustry News
view channel
Nuclear Medicine Set for Continued Growth Driven by Demand for Precision Diagnostics
Clinical imaging services face rising demand for precise molecular diagnostics and targeted radiopharmaceutical therapy as cancer and chronic disease rates climb. A new market analysis projects rapid expansion... Read more