Disposable Dressing Makes Guided IV Insertions Easier
By MedImaging International staff writers
Posted on 11 Sep 2018
A combined sterile barrier and securement dressing facilitates ultrasound-guided peripheral intravenous (UGPIV) insertions.Posted on 11 Sep 2018
The Parker Laboratories (Fairfield, NJ, USA) UltraDrape dressing is designed to address the challenges that clinicians face with UGPIV insertions, including extended procedure time, increased risk of infection and cross contamination, and securement failure resulting from inadequate gel removal. Ultrasound gel is first applied to a removable film layer, enabling target vessel identification while still keeping the sterile puncture area dry and free from gel. The top layer is then discarded, leaving the area dry for successful fixation.
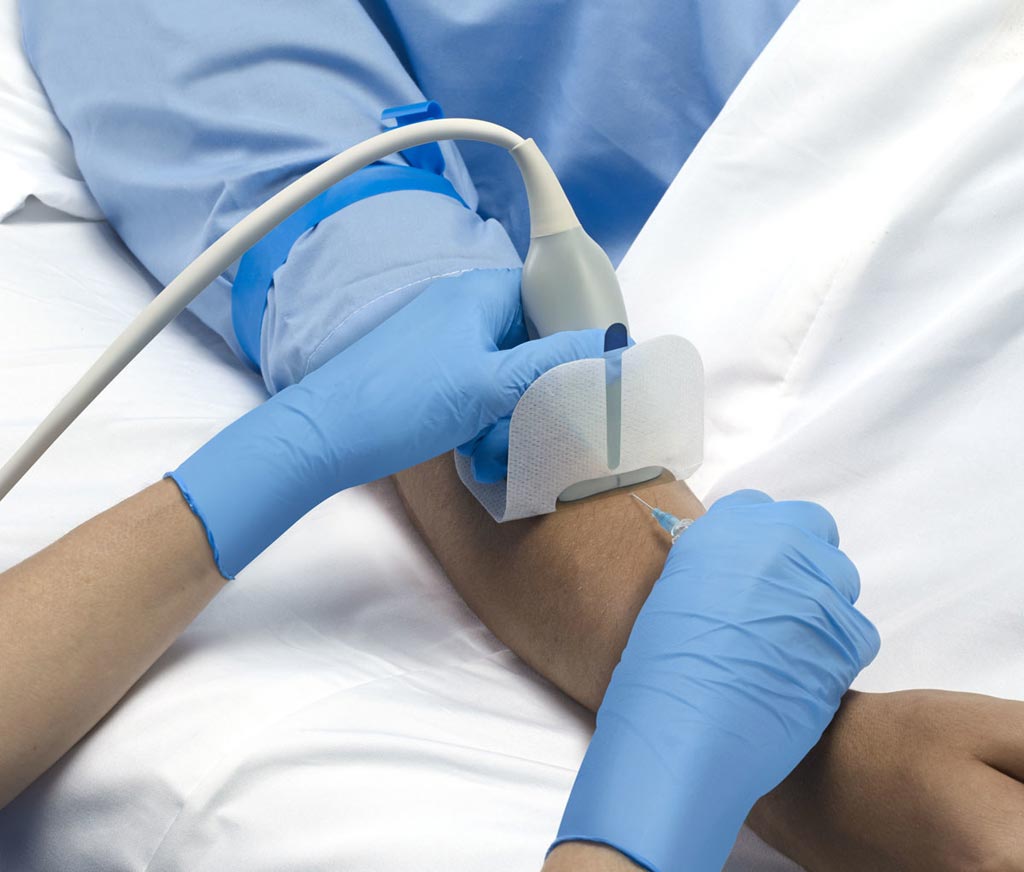
Image: A combination sterile barrier and securement dressing aids UGPIV insertions (Photo courtesy of Parker Laboratories).
The major benefit of UltraDrape is that it eliminates the need to clean the ultrasound transmission gel from the patient’s skin prior to IV securement, saving valuable time. In addition, inadequate removal of transmission gel can lead to dressing failure, requiring more frequent changes and higher contamination rates. The sterile, dual-action UltraDrape dressing is provided in a convenient dispenser containing 50 units, lowering the cost of performing aseptic UGPIV insertions by removing the need for additional securement dressings, sterile gels, and probe covers.
“UltraDrape represents an unprecedented advancement enabling UGPIV practitioners to perform procedures efficiently, effectively, and at a significant cost savings when compared with use of sterile gels and covers,” said Neal Buchalter, president of Parker Laboratories. “We are very excited to make UltraDrape available today through our worldwide distribution network.”
“This combination sterile barrier and securement approach makes it easier and faster to achieve success with ultrasound-guided PIV insertions, and it helps me do a better job as a clinician,” said independent consultant in vascular access Nancy Moureau, RN, PhD. “By not applying gel directly to the patient's skin, I can maintain a better no-touch aseptic technique, which lowers the risk of infection and eliminates time-consuming post-procedure clean up.”
UGPIV is recommended when attempts to obtain peripheral intravenous access by standard methods have failed, and in patients with known difficult PIV access without palpable peripheral vessels. The most common area for UGPIV placement is in the antecubital fossa region, with a high-frequency linear array probe as the recommended ultrasound transducer. UGPIV has been shown to improve IV success rates, decrease the number of percutaneous punctures, and decrease the time required to achieve IV access.