Partnership Intended to Accelerate Development of Clinical Ultrasound Capabilities
By MedImaging International staff writers
Posted on 06 Jun 2016
A life science company and a leading imaging equipment manufacturer have signed a global collaboration agreement for the joint development of new thermo-acoustic enhanced ultrasound technology.Posted on 06 Jun 2016
The new technology can be used with existing ultrasound systems, and enable them to visualize tissue composition and function, and monitor various therapeutic interventions at the point of care. This could lead to broad potential clinical applications such as non-invasive quantification of fat in the liver, and the diagnosis of fatty liver disease.
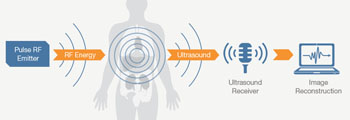
Image: Thermo Acoustic Enhanced UltraSound (TAEUS) Technology enables clinicians to use existing ultrasound equipment for visualizing tissue health, composition, and function at the point of care (Photo courtesy of ENDRA Life Sciences).
Under the agreement GE Healthcare (Chalfont St Giles, Buckinghamshire, UK) will support ENDRA Life Sciences’ (Ann Arbor, MI, USA) efforts to commercialize the Thermo Acoustic Enhanced UltraSound (TAEUS) technology for use in a fatty liver application. GE Healthcare will provide equipment, technical advice, and connect ENDRA to GE clinical ultrasound customers. Under the terms of the agreement ENDRA will give GE Healthcare certain rights of first offer to manufacture and license the target application.
The TAEUS technology is intended to help clinicians make better use of existing ultrasound equipment, at the point of care, and improve patient access, safety, clinical effectiveness, and cost. ENDRA Life Sciences also produces the only available fully 3D imaging solution for imaging anatomy, physiology and labeled molecular targets, called the Nexus-128.
Brian McEathron, VP and GM of GE Healthcare's Radiology and Vascular Ultrasound group, said, "GE has a long history of bringing meaningful innovation to the market in healthcare and other sectors. We believe that ENDRA's technology has the potential to bring significant new capabilities to ultrasound – which aligns well with GE Healthcare's mission of broadening access to high-quality, cost-effective healthcare. We're very excited about working with ENDRA."
Related Links:
GE Healthcare
ENDRA Life Sciences