US FDA Announces Clearance for New Handheld Bladder Scanner
By MedImaging International staff writers
Posted on 11 Feb 2015
A new, portable handheld ultrasound bladder scanner has received United States Food and Drug Administration (US FDA) 510(k) clearance.Posted on 11 Feb 2015
The real-time scanner uses noninvasive, nonionizing ultrasound technology to measure bladder volume. Measuring the bladder volume of a patient with suspected urinary retention problems can prevent unnecessary catheterization and thereby reduce the occurrence of Urinary Tract Infections (UTI), and bring down costs such as those for hospitalization.
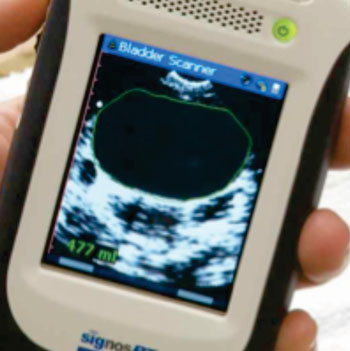
Image: The SignosRT Scanner (Photo courtesy of Signostics).
The SignosRT Bladder scanner is made by Signostics (Clovelly Park, SA, Australia), and is currently sold in Australia and New Zealand, with plans for a European launch in March 2015. The scanner weighs less than 400 g and is intended for dynamic bladder imaging and accurate bladder volume measurements in trauma care, home nursing, midwifery, urology, palliative care, rehabilitation, and for general medical practice.
The real-time scanner has a dynamic range of approximately 120 sB, and a scanning depth of up to 18 cm. The scanner is used with the SIGVIEWER computer software for synchronizing image and patient data and for exporting images.
Related Links:
Signostics
US FDA