Myelin Volume Measurement Tool Receives CE Mark
By MedImaging International staff writers
Posted on 25 Apr 2017
A tool that can be used for rapid quantification of the estimated myelin volume in the brain for diagnostic imaging has been awarded the CE-mark (Conformité Européenne) for clinical use in Europe.Posted on 25 Apr 2017
According to the developer of the post-processing software, the tool is compatible with Magnetic Resonance (MR) scanners from leading vendors.
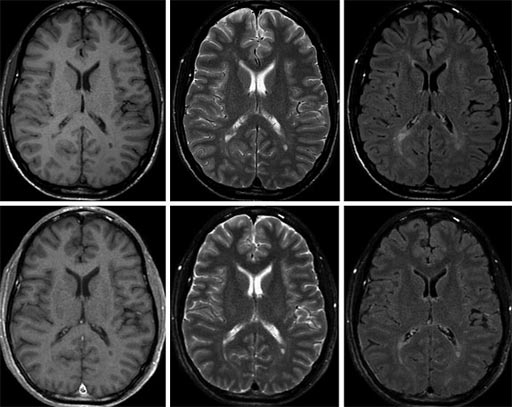
Image: The top images show conventional 1.5T MRI exams, and the lower images show SyMRI exams synthesized in a single scan (Photo courtesy of SyntheticMR).
The Rapid Estimation of Myelin for Diagnostic Imaging (REMyDI) tool is part of the SyntheticMR SyMRI NEURO REMyDI post-processing software package. Myelin quantification enables clinicians to monitor myelin degeneration in patients with neurodegenerative disorders, and diseases that cause brain demyelination. Clinicians can also use the tool to follow myelination in the developing brain. The new REMyDI feature will provide clinicians with automatic myelin volume measurement using data from a single 5-6-minute quantitative MRI scan. Post-processing is automatic and takes less than 10 seconds to complete.
The SyMRI software package enables clinicians to capture multiple image contrasts in one MR scan. The contrast can be adjusted following the scan to create additional images. The system provides automatic segmentation of brain tissue for objective decision support based on quantitative data. Scanner settings can be matched between scans to enable accurate comparison between exams. The images can also be matched with previous images, and conventional protocols. The SyMRI software package is CE-marked, and is pending 510(k) approval from the US Food and Drug Administration (FDA).








 Guided Devices.jpg)