First-Of-Kind Endoscopic Ultrasound Technology Offers Groundbreaking Approach to Patient Safety
Posted on 03 Jan 2024
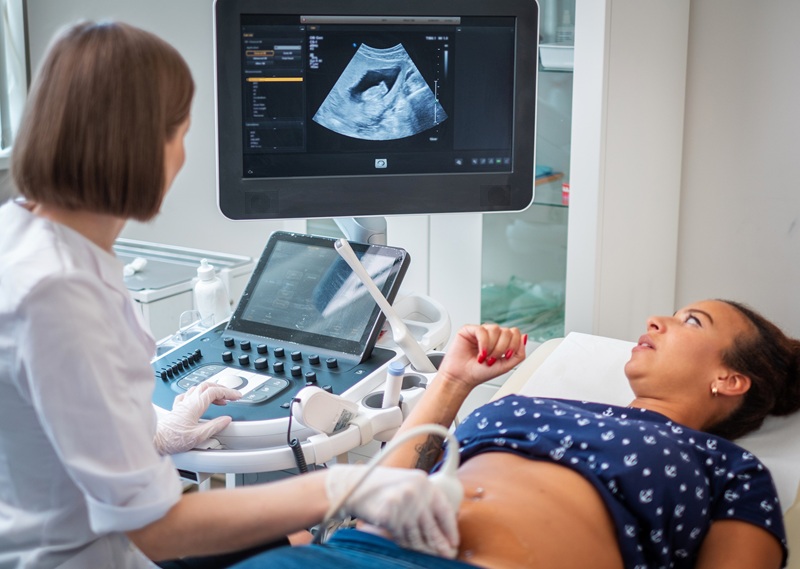
Endoscopic Ultrasound (EUS) is a minimally invasive technique used to diagnose conditions of the gastrointestinal (GI) tract and nearby organs. EUS combines the capabilities of endoscopy and ultrasound to generate detailed visuals of the digestive system, including organs like the pancreas, liver, and gallbladder. Typically, over 95% of EUS procedures are performed in hospital environments, but many patients could benefit from undergoing these procedures in more convenient settings like ambulatory surgery centers (ASC). Now, a first-of-its-kind EUS technology with a groundbreaking approach to patient safety has the potential to shift the site of care to more efficient and preferable settings like ASCs.
The EndoSound Vision System (EVS) from EndoSound Inc. (Portland, OR, USA) is an innovative EUS device designed to attach to upper gastrointestinal endoscopes and seamlessly integrate into the existing ecosystem of any endoscopy center. The EVS marks a substantial advancement in medical technology, particularly targeting the risk of infections linked to the complex cleaning of endoscope elevators. The system is set to redefine EUS with its affordability, enhanced safety, and capability to convert a standard upper GI endoscope into an EUS system, thereby broadening access to this vital diagnostic and therapeutic procedure.

The FDA has granted 510(k) clearance to the EVS, after recognizing it as a Breakthrough Device in July 2021, highlighting its innovative approach to patient safety, cost-effectiveness, and expanding access to essential healthcare services. This FDA endorsement confirms the system's safety and efficacy, reinforcing EndoSound's dedication to advancing healthcare through innovative solutions.
"We are thrilled to receive 510(k) clearance for our EVS, a testament to the dedication and innovation of the entire EndoSound team," said Dr. Stephen Steinberg, President and CEO at EndoSound. “This milestone underscores our commitment to advancing medical technology and improving patient outcomes. With the EVS, we aim to not only enhance the safety of endoscopic procedures but also contribute to expanding access to care for patients worldwide.”
Related Links:
EndoSound Inc.










 Guided Devices.jpg)