MRI-Guided Focused Ultrasound System Burns Breast Cancer Tumors without Surgery
Posted on 11 Oct 2023
In a significant breakthrough in the field of non-invasive treatments for breast cancer, scientists have developed a technology capable of eliminating tumors without the need for surgical intervention, all within an outpatient setting.
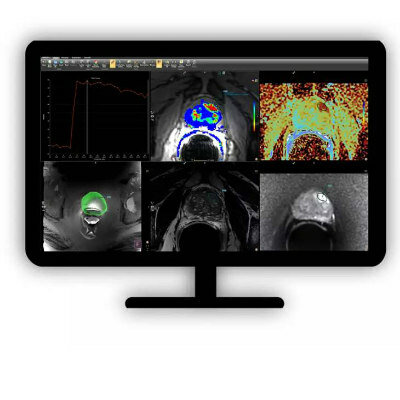
The MUSE breast cancer treatment system developed by researchers from the Huntsman Cancer Institute at University of Utah Health (Salt Lake City, UT, USA) employs a two-step, non-surgical approach for locating and ablating, or removing, breast tumors. Initially, magnetic resonance imaging (MRI) is utilized to identify and pinpoint the tumors. Once the tumors are located, focused ultrasound technology is used to heat and subsequently destroy the cancerous cells. The entire treatment, which allows the patient to stay awake, takes about 90 minutes and is completed within a few hours.

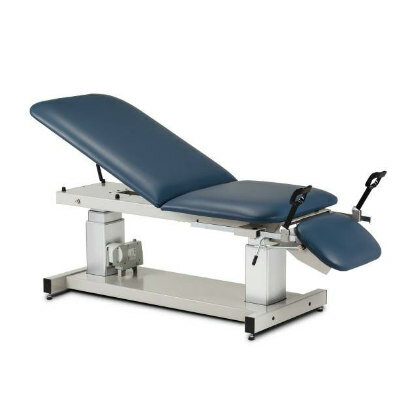
Designed with patient comfort in mind, the MUSE system incorporates a special table for use during the MRI. The specialized device focuses ultrasound waves into a specific area of the tumor. These waves heat the cancerous tissue in intervals of 30 seconds, and are directed to cover the whole tumor. Currently in its phase 1 clinical trials, the first patient in the U.S. was treated using the MUSE system in a pioneering clinic trial in February 2023. The research team anticipates that it will take at least five years to advance through phase 3 clinical trials before MUSE becomes commercially available as a surgery-free option for treating breast cancer.
“The purpose of the current study is to see how well patients tolerate the ablation, basically how much pain they have during the procedure,” said Cindy Matsen, MD, breast surgeon at Huntsman Cancer Institute, who is the principal investigator for this clinical trial. “We’ll also be looking at the tissue after surgery to see how effective the ablation was. Only 50% is being ablated at this point to make sure we can still do tests that may be needed on the tumor afterwards. In the future, we hope this ablation will be effective enough to replace surgery for some women.”
Related Links:
University of Utah Health