Micro-Ultrasound System Receives CE Mark for Prostate Imaging and Biopsies
By Andrew Deutsch
Posted on 07 Dec 2016
A manufacturer of high-resolution micro-ultrasound systems has received the CE Mark (Conformité Européenne) approval for its prostate imaging and biopsy guidance systems.Posted on 07 Dec 2016
The micro-ultrasound platform provides a real-time resolution down to 70 microns for prostate imaging and biopsies, and operates at a frequency of 29 MHz, much higher than that of conventional ultrasound systems. The increased resolution provides urologists with improved visualization of suspicious regions, and enables them to perform targeted biopsies of the prostate at the same time.
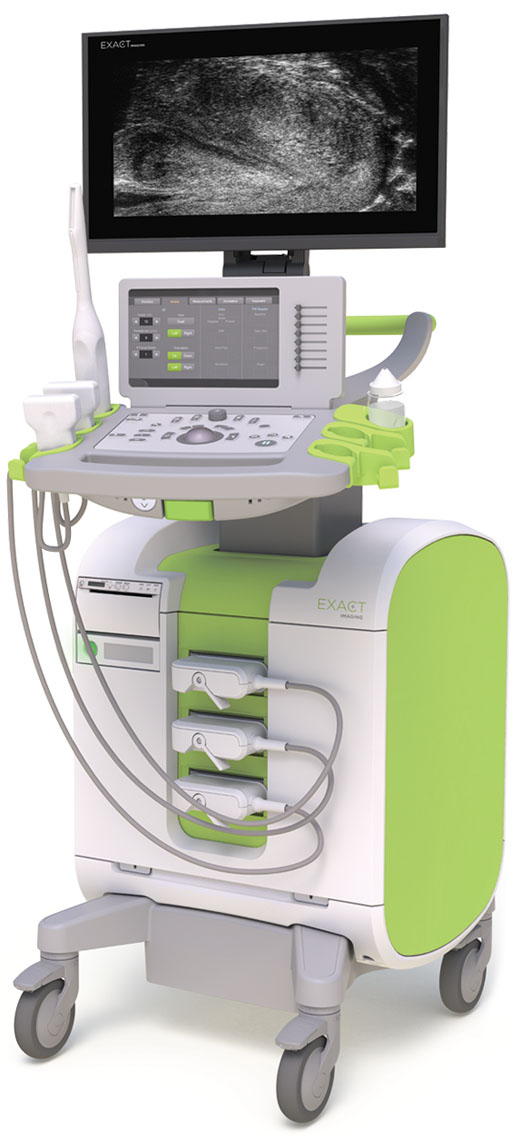
Image: The ExactVu Micro-ultrasound system (Photo courtesy of Exact Imaging).
The ExactVu system was developed by Exact Imaging (Toronto, Canada), a world leader in the development of high-resolution micro-ultrasound systems. Following the CE Mark approval the company plans to begin commercial sales and expand activity into Europe. Exact Imaging clinical applications, technical support, and direct sales will be based in Belgium.
CEO and President of Exact Imaging, Randy AuCoin, said, “We are thrilled with the achievement of CE Mark approval for our ExactVu system, which is the culmination of many years of technical development. There is significant built-up demand for acquiring the ExactVu instrument and this approval will allow us to be able to commercialize our platform in Europe. With the ExactVu platform, urologists will have a new level of real-time resolution to facilitate actually targeting their prostate biopsies at suspicious regions. The goal is to provide the best tools to help the urologist make the most informed decisions and ultimately, to improve patient outcomes.”
Related Links:
Exact Imaging








 Guided Devices.jpg)