US FDA Clears Smart Mobile-Connected High-Resolution Bladder Scanner
By MedImaging International staff writers
Posted on 18 May 2016
A global medical device manufacturer has received US FDA clearance for a new urologic visualization device that can improve point-of-care clinical decision-making.Posted on 18 May 2016
The device is the first mobile-connected ultrasound visualization device targeted at urologic care to receive US Food and Drug Administration (FDA) 510(k) clearance.
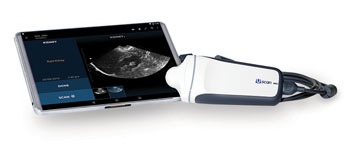
Image: The Uscan smart mobile-connected high-resolution bladder scanner is now cleared by the US FDA (Photo courtesy of Signostics).
The Uscan device developed by Signostics (Bothell, WA, USA) has a removable probe, a high-resolution tablet with a touch screen, and handheld displays. The Uscan is compatible with the Android operating system, features built-in WiFi and Bluetooth connectivity, and is interoperable with Electronic Health Record (EHR) systems. The device does not require annual calibration, and provides real-time user guidance.
The Uscan can acquire up to 256 bladder slices, and uses algorithms to actively recognize bladder contours in 3D providing accurate volume measurements. The device can also be used for real-time ultrasound imaging of the bladder stones, gallbladder, prostate, pelvic floor, kidneys, for the placement of catheters, and for rapid visual tracking, and observation. The device can also be used in emergency departments, maternity wards, for pediatrics, oncology, rehabilitation, in home nursing, and in geriatrics.
CEO of Signostics, Kevin Goodwin, said, “Uscan doesn’t just scan; it sees – providing intelligent urologic visualization by leveraging science from current-day computer vision algorithms aimed at more efficient and confident point-of-care clinical decision-making. Uscan will exceed historical industry standards for bladder volume measurement accuracy yet will also enable use for other urologic imaging needs, reducing the delays and expense of engaging specialized ultrasound equipment or sonographers.”
Related Links:
Signostics