Rugged, Portable Ultrasound System Receives Regulatory Approval
By MedImaging International staff writers
Posted on 08 Mar 2016
A new portable ultrasound system with improved transducer signal penetration, and contrast resolution has been released following US and EU regulatory approval.Posted on 08 Mar 2016
The portable system, designed for the acute-care environment, features transducers wrapped in a metal housing to prevent accidental damage, increased penetration and contrast resolution, a new wide-angle display with a 33% higher viewing angles, armored cables, and a water-resistant control panel.
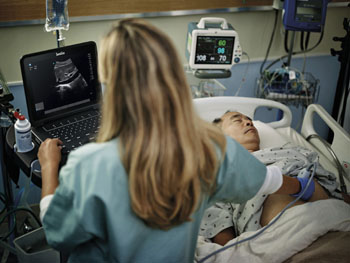
Image: The FujiFilm SonoSite Edge II with DirectClear techology (Photo courtesy of Business Wire).
The Edge II which uses DirectClear techology, was developed by FujiFilm SonoSite (Bothell, WA, USA). The system received was approved for use in the US and in Europe after receiving US Food and Drug Administration (FDA; Silver Spring, MD USA) 510(k) clearance, and the CE marking. FujiFilm SonoSite is the world leader in bedside and point-of-care ultrasound, and an industry leader in ultra-high frequency micro-ultrasound technology.
Diku Mandavia, MD, FACEP, FRCPC, chief medical officer, senior vice president, FUJIFILM SonoSite and FUJIFILM Medical Systems USA, said, “Since SonoSite introduced the first portable ultrasound system in 1999, it has continued to build solutions that anticipate the bedside provider’s needs. The Edge II ultrasound system stays true to the SonoSite legacy of durability, reliability, and ease of use. However, we also incorporated enhancements to accelerate the time to image acquisition, enabling clinicians to make more confident decisions and focus on what matters most, the patient.”
Related Links:
FujiFilm SonoSite