Handheld Ultrasound Scanners Receive Market Clearance
By MedImaging International staff writers
Posted on 21 May 2013
Novel handheld ultrasound scanners features include patented technology and an award-winning design to provide a lightweight, handheld ultrasound device for high-quality imaging at the point-of-care for healthcare professionals, medical practitioners, and veterinarians worldwide across a wide range of clinical settings.Posted on 21 May 2013
Handheld ultrasound device manufacturer Signostics (Thebarton, SA, Australia) has received a key boost with 510K clearance from the US Food and Drug Administration (FDA) to market its new Signos RT to the American market.
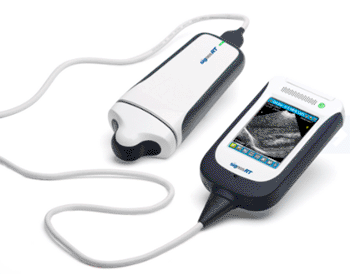
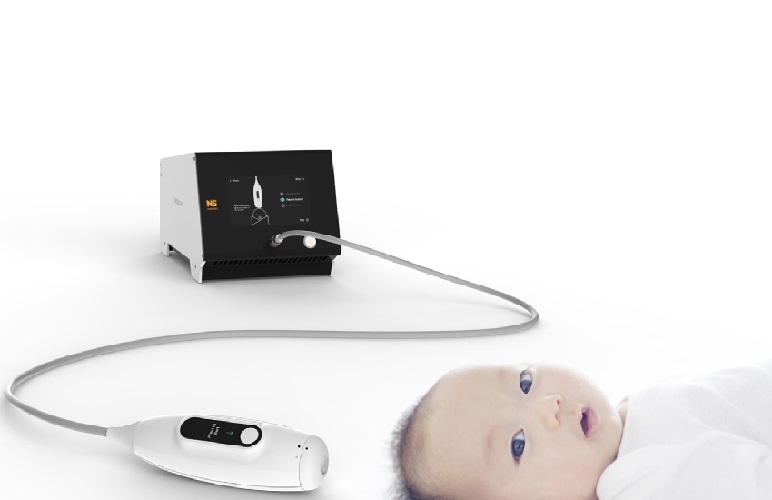
Image: The Signos RT/Sonimage P3 device (Photo courtesy of Signostics).
510(k) clearance from the FDA means the Signos RT can now be marketed throughout the US where it will be marketed as the Sonimage P—the brand name of Signostics’ newly appointed global partner Konica Minolta, Inc. (Tokyo, Japan).
“FDA clearance for the USA market further enhances the potential of this innovative product and will enable medical practitioners and patients throughout America to benefit from the real-time point-of-care imaging capabilities that the device provides,” said Signostics’ chief executive officer Warren Ortmann. “This clearance will help us build on the enormous interest we have already received from potential distribution partners throughout Europe, South East Asia, and our own Australian market who have seen and experienced the device first-hand. It certainly is a very important and exciting milestone for Signostics and is a fantastic boost for our new distribution partnership with Konica Minolta.”
The Signos RT/Sonimage P3 device features innovative patented technology and an award-winning design to provide a lightweight, handheld ultrasound device for high quality imaging at the point-of-care for medical practitioners, healthcare professionals, and veterinarians globally across a wide range of clinical settings.
Earlier in 2013, Signostics and Konica Minolta reported a major distribution partnership that sees Konica Minolta marketing the Signos RT/Sonimage P3 device exclusively through Japan, India, China, and the United States.
Related Links:
Signostics