Digital IVUS Catheter Receives US and European Clearance
By MedImaging International staff writers
Posted on 23 Oct 2012
A new intravascular ultrasound (IVUS) catheter is designed to image large vessels and aid in the use of less iodinated contrast in endovascular procedures.Posted on 23 Oct 2012
Volcano Corp. (San Diego, CA, USA), a developer and manufacturer of precision-guided therapy tools devised to enhance the diagnosis and treatment of coronary and peripheral vascular disease, reported it has received clearance to market its new Visions PV .035 digital IVUS catheter in both the United States and Europe.
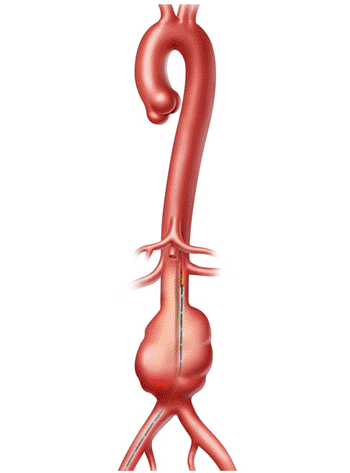
Image: The Visions PV .035 digital IVUS catheter (Photo courtesy of Volcano).
Intravascular ultrasound imaging of diseased arteries and veins allows physicians to see vessel morphology and perform real-time measurements that influence treatment strategies and therefore patient outcomes. The Visions PV .035 catheter integrates new centimeter markers to facilitate length measurements critical for choosing treatment devices.
“Endovascular repair is preferred over open surgical repair by many patients because of its less invasive nature, shorter recovery times, and lower complication rates for patients with suitable anatomy,” said David Sheehan, chief operations officer and intravascular imaging business-unit leader of Volcano Corp. “However, precise device sizing and placement is critical for positive outcomes. Volcano believes IVUS can play an important role in guiding endovascular and other peripheral therapies. The development of this new catheter is just one example of our advancing commitment to this area. Another challenge in endovascular repair is renal insufficient patients. These patients are susceptible to acute kidney injury--a condition associated with high cost, morbidity, and mortality--due to intraoperative use of iodinated contrast. Using our Visions PV .035 catheters with new centimeter markers reduces the amount of contrast needed, thus minimizing risk and creating more options for these patients.”
Scott Huennekens, president and chief executive officer of Volcano Corp. added, “Our share leadership in worldwide intravascular and peripheral imaging is because we innovate products that are clinically relevant and improve outcomes for our physicians. Moreover, we continue to add new and improved peripheral products that all work on our existing multimodality platform.”
Company spokespersons pointed to a study conducted in Osaka, Japan, published in the European Journal of Vascular and Endovascular Surgery that demonstrated the use of IVUS resulted in considerably less contrast agents required during endovascular aortic repair (EVAR). The new centimeter markers make these procedures simpler and quicker, and eliminate the need for additional marker catheters or a sterile ruler.
The new catheter features other improvements, new materials, a longer connector length to improve procedural flexibility, a softer, more rounded tip with lower entry profile and GlyDx hydrophilic coating for delivery through tortuous anatomy.
Related Links:
Volcano