Automated Breast Ultrasound System Receives FDA Approval
By MedImaging International staff writers
Posted on 24 Sep 2012
An automated breast ultrasound system is first device approved specifically for cancer screening of women with dense breasts in United States, Canada, and Europe.Posted on 24 Sep 2012
U-Systems (Sunnyvale, CA, USA), a developer of automated breast ultrasound system, announced that the somo•v automated breast ultrasound (ABUS) system has been approved by the US Food and Drug Administration’s (FDA) for breast cancer screening as an adjunct to mammography for asymptomatic women with dense breast tissue. With the approval, the somo•v ABUS system becomes the only device approved specifically for screening women with dense breasts.
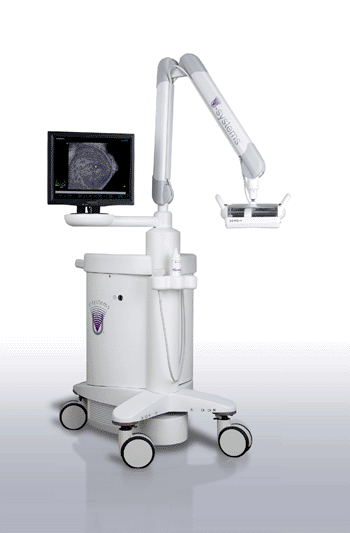
Image: The somo•v ABUS device (Photo courtesy of U-Systems).
“The FDA approval of the somo•v ABUS device is an exciting and important development in the detection of early, curable breast cancer. The use of somo•v for screening women with dense breasts will undoubtedly have a positive impact for women currently being underserved by mammography,” said Rachel Brem, MD, director of breast imaging at The George Washington University Hospital (Washington DC, USA). “Mammography is an effective tool at finding breast cancer, but it doesn’t work equally well in everyone. Recently completed studies demonstrated [that] with the addition of ABUS we find about 30% more cancers in women who have normal mammogram, normal physical examination, and dense breasts. For the more than 40% of women who have dense breasts, this is a significant advancement in their breast healthcare.”
FDA approval comes after a Radiological Devices Panel of the FDA’s Medical Devices Advisory Committee unanimously recommended approval of U-Systems’ premarket approval (PMA) application in April 2012. The somo•v ABUS system is the only ultrasound device approved for breast cancer screening in the United States, Canada, and 27 European Union countries as an adjunct to mammography for asymptomatic women with dense breast tissue.
“We are proud to announce that the somo•v ABUS system has received FDA approval for breast cancer screening and U-Systems can offer radiologists and breast imaging experts an important new tool to improve early detection in women with dense breasts,” said Ron Ho, president and CEO of U-Systems. “Enabling radiologists to use the information obtained from mammography and integrate that with the information obtained with ultrasound, leverages the potential of ABUS in a screening environment to find the 30% additional cancers that would not have been found with mammography alone. Research shows that ABUS can help find cancer in women with dense breasts, and that the cancers are smaller and early stage. With formal approval, we are moving rapidly from development to commercialization and look forward to making the somo•v ABUS system more widely available across the United States.”
Dense breast tissue not only increases the risk of breast cancer up to four to six times but also makes cancer more difficult to detect using mammography, according to multiple large studies. One study, published in 2007 in the New England Journal of Medicine (NEJM), showed 35% of breast cancer goes unidentified by mammography in women with dense breasts because density masks the appearance of tumors. As breast density goes up, the accuracy of mammograms declines.
Using proprietary technology to automate the ultrasound imaging process, the U-Systems’ somo•v ABUS system was developed specifically for the high-volume, breast cancer screening environment. The somo•VIEWer Advanced three-dimensional (3D) Workstation enables fast, accurate review, and archiving of patient exams, optimizing breast ultrasound screening workflow.
Related Links:
U-Systems