Low Doses of Radiation Promote Tumorigenic Cells
By MedImaging International staff writers
Posted on 30 Jul 2019
A new study reveals that low-dose ionizing radiation (LDIR; equivalent to three CT scans) gives cancer-capable cells a competitive advantage over normal cells.Posted on 30 Jul 2019
Researchers at the University of Cambridge (United Kingdom) and Wellcome Sanger Institute (Hinxton, United Kingdom) conducted a study to examine how oxidative stress resulting from LDIR affected wild-type and p53 mutant cells present in the transgenic mouse esophagus. As the researchers speculated that altering the selective pressure on mutant cell populations may cause them to expand or contract, they also gave the mice N-Acetyl Cysteine (NAC), an antioxidant, before exposure to the same level of radiation.
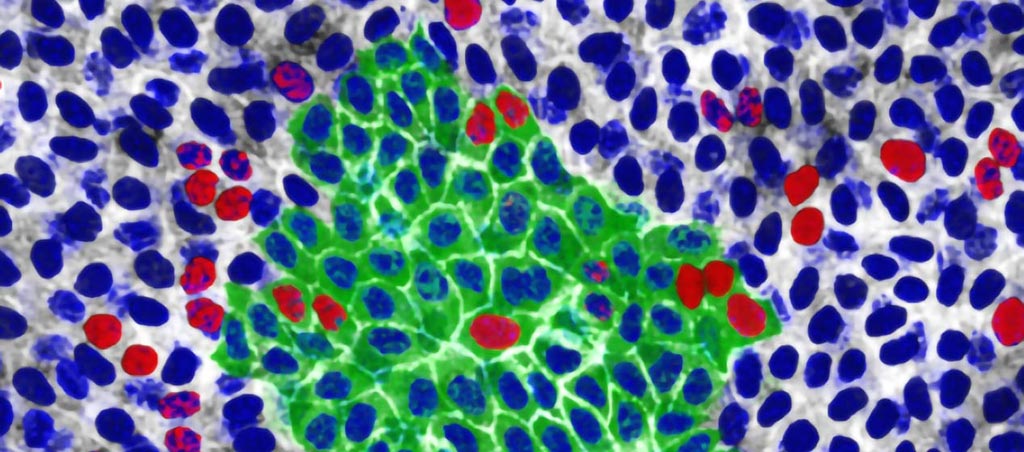
Image: p53 mutant cells (red and green) expansion in mouse esophageal tissue (Photo courtesy of Wellcome Sanger Institute).
The researchers found that LDIR drives wild-type cells to stop proliferating and differentiate, and that p53 mutant cells are insensitive to LDIR and outcompete wild-type cells following exposure. However, they found that combining NAC antioxidant treatment and LDIR reverses this effect, promoting wild-type cell proliferation and p53 mutant differentiation. The researchers suggest that NAC redox and radiation combined could be used to deplete p53-mutant cells from the normal esophagus by, altering the mutational landscape of an aging tissue. The study was published on July 18, 2019, in Cell Stem Cell.
“Our bodies are the set of 'Game of Clones' - a continuous battle for space between normal and mutant cells,” said lead author David Fernandez-Antoran, PhD, of the Wellcome Sanger Institute. “We show that even low doses of radiation, similar to three CT scans' worth, can weigh the odds in favor of cancer-capable mutant cells. We've uncovered an additional potential cancer risk as a result of radiation that needs to be recognized.”
“Giving mice an antioxidant before exposing them to low doses of radiation gave healthy cells the extra boost needed to fight against the mutant cells in the esophagus and make them disappear,” added study co-author Kasumi Murai, PhD, of the Wellcome Sanger Institute. “However, we don't know the effect this therapy would have in other tissues. It could help cancer-capable cells elsewhere become stronger. What we do know is that long term use of antioxidants alone is not effective in preventing cancer in people.”
p53 is a strong tumor suppressor because it lies in the midst of a signaling hub, with many different signals, such as oncogene activation, DNA damage, mitotic impairment, or oxidative stress adjusted by p53 to initiate the correct transcriptional response to eliminate cancer-prone cells from the replicative pool. In approximately 50% of human cancers p53 is mutated and in many of the remaining cases, the function of the retained wild type p53 protein is compromised.
Related Links:
University of Cambridge
Wellcome Sanger Institute














