Injectable Spacer Reduces Radiotherapy Consequences
By MedImaging International staff writers
Posted on 24 Jun 2015
A novel spacer separates the rectum from the prostate during radiotherapy (RT), potentially reducing the risk of adjacent organ-at-risk (OAR) injury. Posted on 24 Jun 2015
The SpaceOAR system is a temporary injectable gel that protects the rectum in men undergoing RT for prostate cancer. The hydrogel, which is created from a mixture of two separate syringes, is injected through the perineum, guided by transrectal ultrasound. The material flows into the space between the prostate and the rectum and expands within ten seconds, filling the space and reducing rectum radiation during prostate RT. The hydrogel remains in place for three months, and is then liquefied and absorbed, leaving nothing behind.
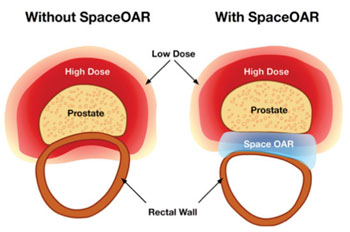
Image: The SpaceOAR system benefits (Photo courtesy of Augmenix).
Because the prostate gland is located close to the bladder and rectum, it is important for RT to be tightly focused on the prostate to avoid serious side effects to surrounding organs, such as bleeding, diarrhea, and pain. Shielding the rectum from radiation also permits dose escalation and hypo-fractionation, resulting in more prostate radiation, improved cancer kill rates, and fewer radiation treatment sessions. The SpaceOAR system is a product of Augmenix (Waltham, MA, USA), and has been approved by the US Food and Drug Administration (FDA).
“For years, hydrogel products have been used safely to protect the most sensitive parts of the body as sealants and adhesion barriers, and now prostate cancer patients will also be able to benefit,” said John Pedersen, CEO of Augmenix. “FDA clearance of the SpaceOAR System represents a significant development in advancing the safety, precision, and flexibility with which prostate cancer radiotherapy can be delivered.”
Related Links:
Augmenix