Hybrid OR Imaging System Moves Along Defined Pathways, Guided by Laser
By MedImaging International staff writers
Posted on 22 Aug 2012
A hybrid operating room (OR) imaging system is an innovative new laser-guided system that has been designed to capture the advantages of both floor- and ceiling-mounted systems, without the limitations of either. Posted on 22 Aug 2012
GE Healthcare (Chalfont St. Giles, UK) has already received six orders for its Discovery IGS 730 since receiving US Food and Drug Administration (FDA) clearance in February 2012. The system uses the same digital flat-panel detector technology as GE Innova interventional imaging systems. However, instead of being fixed in position, the imaging device is on mobile gantry that moves along user defined pathways, guided by laser.
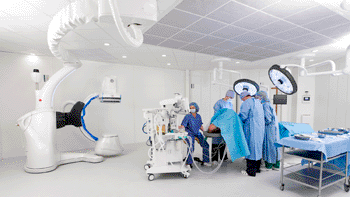
Image: The Discovery IGS 730 system (Photo courtesy of GE Healthcare).
At the touch of a button, clinicians can move the system to the table for imaging various anatomies, and then move it aside and place it, allowing room to position physicians, nurses, technologists, anesthesiologists, and other personnel optimally for surgery, with unobstructed access to the patient.
St. Luke’s University Hospital (Bethlehem, PA, USA), the first to place an order world-wide, plans to install the system over the coming months. The Discovery IGS 730 is designed to provide high precision imaging with complete patient access, and optimal in-room mobility to accommodate a wide range of procedures--from complicated interventional to surgical procedures.
Dr. Hal Folander, chairman of the radiology department and section chief of interventional radiology at St. Luke’s University Health Network, participated in early evaluations of the Discovery IGS 730. “After initially viewing the power of this system it was clear that this is an advancement that is on-par with the invention of flat panel technology for interventional procedures,” said Dr. Folander. “At St. Luke’s, we are constantly looking for solutions and systems that help us improve the efficiency of our procedures as well as the experience for our patients. The Discovery IGS system will not only help our providers access patients better during procedures, but will allow us to provide our patients a revolutionary development in imaging and quality care.”
The Discovery IGS 730 is a first-of-its kind system in imaging, as it is neither floor- nor ceiling-mounted, but enables full patient access without the need to suspend the system above the patient. It has the mobility of a C-arm with the power and image quality of a fixed system. This laser-guided, motorized mobile gantry creates an interventional environment without boundaries. It allows complete access to the patient and unlimited parking capability, while creating sterility for a flexible and secure OR environment. The unique gantry has a new wide bore design, which allows for steep angles, ease in three-dimensional (3D) acquisition, especially for large patients.
“We are extremely encouraged with how clinicians around the world, like those at St. Luke’s, have responded to the breakthroughs in our Discovery IGS 730. In addition to the five orders we have received to date, we have interest from over 100 other customers from around the world. With a breakthrough new technology, you sometimes see longer adoption curves, but when you consider the global market for premium interventional systems, this is truly a rapid ramp-up. We knew we had something special as we were designing the system. It’s nice to see it being validated by our customers,” said Hooman Hakami, president and CEO, detection and guidance solutions (DGS), GE Healthcare.
The Discovery IGS 730 is cleared by the US FDA.
Related Links:
GE Healthcare














