3D Digital Mammography Tomosynthesis System Receives US Approval
By MedImaging International staff writers
Posted on 13 Dec 2010
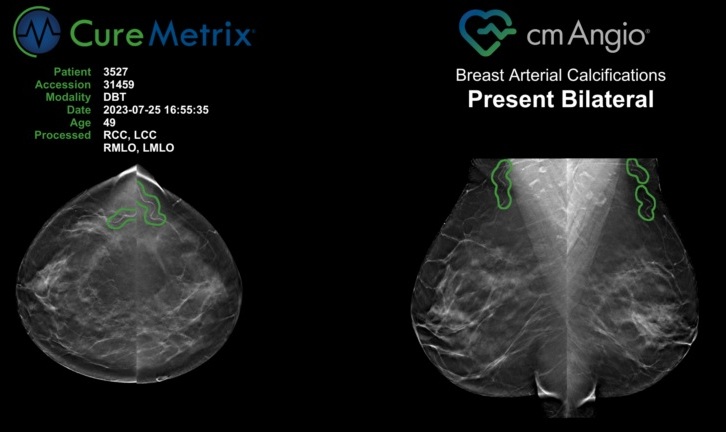
A three-dimensional (3D) digital mammography system is a new application for breast cancer screening and diagnosis. Unlike earlier generation mammography systems that produce two-dimensional images, breast tomosynthesis generates 3D images that are designed to reveal the inner architecture of the breast, free from the distortion typically caused by tissue shadowing or density. Posted on 13 Dec 2010
Hologic, Inc. (Bedford, MA, USA), a developer, manufacturer, and supplier of diagnostics products, medical imaging systems and surgical products focused on the healthcare needs of women, announced that Hologic has received an approvable letter from the US Food and Drug Administration (FDA) for the Selenia Dimensions 3D digital mammography tomosynthesis system. Final approval of the company's premarket approval (PMA) application for the system remains subject to satisfactory review and inspection of our manufacturing facility, methods and controls. The company plans to work closely with the FDA to complete this final inspection.
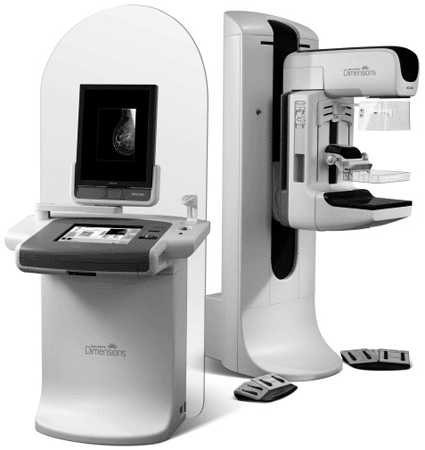
Image: The Selenia Dimensions 3D digital mammography tomosynthesis system (photo courtesy Hologic).
"We are extremely pleased to have received the FDA's approvable letter, which represents an important step forward in the commercialization of our Selenia Dimensions tomosynthesis system,” said Rob Cascella, president and chief executive officer. "The Selenia Dimensions technology is designed to provide radiologists with enhanced screening and diagnostic capabilities through the incorporation of fast, high-quality 3D imaging in combination with 2D imaging. We believe this new technology will address many of the limitations present in stand-alone 2D imaging and improve upon both sensitivity and specificity. We look forward to working with the FDA to complete the remaining steps in the approval process.”
Hologic's Selenia Dimensions 3D system is available commercially in more than a dozen countries outside the United States, including countries in Europe, Latin America, and Asia. In North America, commercial systems are installed in Canada and Mexico. Hologic management held a press conference to discuss Selenia Dimensions technology on Monday, November 29, 2010, at the Radiological Society of North America (RSNA) annual meeting in Chicago, IL, USA.
Related Links:
Hologic




 Guided Devices.jpg)