U.S. Plans Production of Medical Isotopes in 2018
By MedImaging International staff writers
Posted on 26 Apr 2017
Once operational, the University of Missouri Research Reactor will be capable of supporting nearly half of U.S. demand for molybdenum-99 (Mo-99), which currently must be imported from outside North America.Posted on 26 Apr 2017
MURR and its partners Nordion and General Atomics have announced that MURR’s license amendment request (LAR) has been submitted to the U.S. Nuclear Regulatory Commission, a critical step towards implementing domestic U.S. production of Mo-99. Once approved, MURR will begin producing Mo-99 using selective gaseous extraction (SGE), a proprietary technology developed by GA to extract the isotope from low enriched uranium targets.
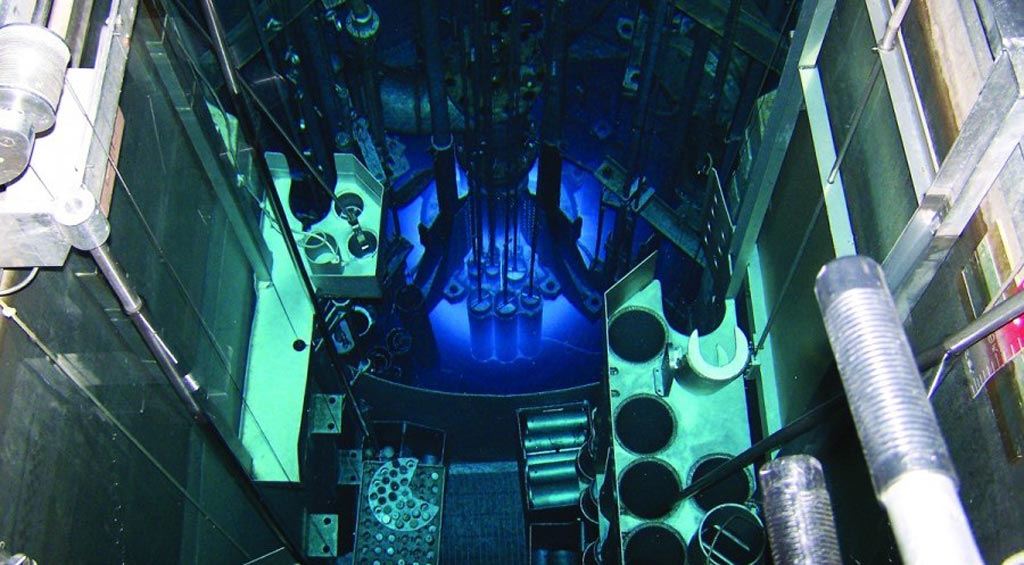
Image: The University of Missouri Research Reactor (Photo courtesy of MURR).
The patented approach will produce Mo-99 of high specific activity, while avoiding the production of liquid uranium waste, a significant problem with existing technologies that require highly enriched uranium (HEU). The extracted Mo-99 will be sent to Nordion for final purification and distribution to radiopharmaceutical manufacturers, after which it will be distributed to hospitals and medical facilities around the world. Nordion will continue to maintain its conventional Mo-99 processing capacity through March 31, 2018, in the event of a significant global shortage of Mo-99.
“This LAR submission shows the Nuclear Regulatory Commission that we will have all of the technology, expertise, and safety measures needed to begin producing Mo-99 in place and ready to go once approval has been received,” said Ralph Butler, executive director of MURR. “As a public research institution, we are proud to play a partnership role with GA and Nordion in helping America secure a new, domestic source of Mo-99.”
The most important medical isotope, technetium-99m (Tc-99m), is obtained from the decay of its parent Mo-99, and is used in more than 80% of all nuclear medicine procedures. Mo-99 is packed into source containment vessels and distributed to hospitals, where nuclear medicine specialists can draw off the Tc-99m as needed for about a week. Because of its unstable nature, Mo-99 does not occur naturally and is traditionally produced using HEU in research reactors in Canada, the Netherlands, Belgium, France, Australia, and South Africa.