New Immuno-PET Imaging Technique Identifies Glioblastoma Patients Who Would Benefit from Immunotherapy
Posted on 04 Nov 2024
Glioblastoma is a type of brain tumor associated with a very poor prognosis, with average survival rates of 12 to 18 months and only 5% of patients surviving beyond five years. Research has shown that certain patients, particularly those with aggressive tumors, may respond favorably to immunotherapy drugs; however, there is currently no method to assess this without a tumor biopsy. Elevated levels of the PD-L1 protein have been detected in rapidly progressing glioblastoma tumors. This protein functions as a brake on the immune system, and targeting PD-L1 to inhibit its activity could potentially reactivate the immune response against the cancer. Historically, a biopsy has been the sole method for evaluating PD-L1 levels in brain tumors. However, biopsies provide only a static snapshot of protein levels at the time of sampling, and there can be a significant delay in treatment decisions, during which protein levels may fluctuate. Due to the risks associated with infection and bleeding, biopsies are seldom performed for glioblastomas before surgery to remove the tumor, leaving many patients without access to potentially beneficial treatments. Consequently, the difficulties in assessing PD-L1 levels without a biopsy have led to the exclusion of patients with newly diagnosed primary brain tumors from early-phase clinical trials. A new imaging technique may now allow patients with aggressive brain tumors to access cutting-edge immunotherapy treatments.
Researchers at The Institute of Cancer Research (London, UK) have developed a novel immuno-PET imaging technique that could identify which glioblastoma patients are likely to benefit from immunotherapy and track their response over time. They created a radiotracer—a radioactive molecule linked to an antibody—that specifically binds to the PD-L1 protein, enabling measurement of its levels in glioblastoma patients. Findings published in the journal Neuro-Oncology demonstrated that the radiotracer effectively binds to PD-L1 on tumor and immune cells, as seen in PET scans. Eight newly diagnosed glioblastoma patients received the tracer intravenously, followed by PET scans at 48 and 72 hours post-injection. The PET scans revealed successful binding of the tracer to PD-L1 positive cells in the tumor and throughout the body. These findings were then compared with biopsies collected during surgical tumor removal.
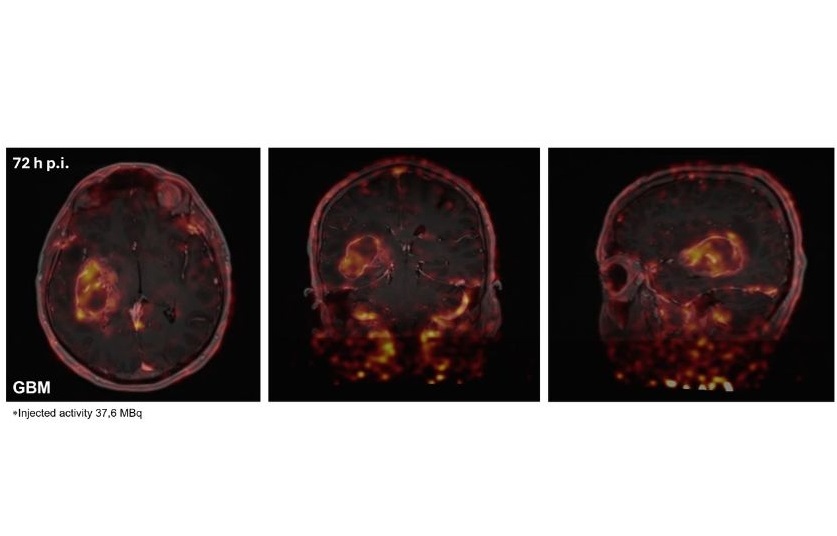
Among the patients, five were randomly selected to receive pembrolizumab prior to surgery. Pembrolizumab is a monoclonal antibody that inhibits PD-L1 by targeting its interaction with a protein called PD-1. The researchers observed lower levels of the tracer in the tumors of these patients, suggesting that the drug effectively acts on the PD-L1 protein, thus removing the immune system's inhibitory effects and allowing it to combat the cancer. Additionally, these patients showed increased tracer levels in lymph tissues, indicating that the drug was activating immune cells throughout the body. Notably, three of these five patients experienced stabilization of their cancer without further growth. The researchers plan to investigate the relationship between the patients' responses to the drug and the levels of PD-L1 in their tumors prior to treatment. The clinical trial aims to enroll 36 glioblastoma patients to assess the effectiveness of pembrolizumab administered before surgery, as well as to evaluate whether PET imaging with the radiotracer can be used to monitor progress and adjust treatment as necessary. Furthermore, the team has developed an alternative radiotracer that may prove even more effective than the antibody used in this study. This smaller molecule is expected to pass through the blood-brain barrier more easily, allowing for PET scans to be performed just one hour after injection. The researchers are hopeful about testing this new molecule in similar studies in the future.
“This study could revolutionize glioblastoma treatment, as we’ve shown that it is possible to image an immunotherapy target with our radiotracer. Being able to take a scan of the patient’s body and see the levels of this target means that we can predict the patients’ response, see their immune system responding to the treatment, and alter treatment where necessary – providing a personalized treatment plan based on the unique characteristics of their tumor, all without the need for a pre-surgery biopsy,” said Dr. Gabriela Kramer-Marek, Associate Professor and Group Leader in Preclinical Molecular Imaging at The Institute of Cancer Research. “I look forward to seeing the results of our larger clinical trial to assess how effective this immunotherapy is in glioblastoma patients – and I hope that our radiotracer will tell us more about the biology behind why some tumors are more responsive than others.”
Related Links:
The Institute of Cancer Research














