FDA Approves MR-Conditional Pacemaker
By MedImaging International staff writers
Posted on 28 Aug 2017
A new, small, MR-conditional cardiac resynchronization therapy pacemaker is now commercially available following US FDA approval.Posted on 28 Aug 2017
The device has a volume of only 15 cc and can last nearly 10 years, reducing the number of device replacements needed by heart failure patients.
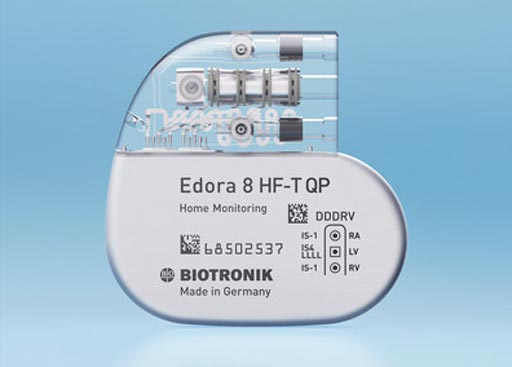
Image: The new Edora HF-T QP MR conditional quadripolar, cardiac resynchronization therapy pacemaker (Photo courtesy of BIOTRONIK).
The Edora HF-T QP device was developed by BIOTRONIK (Lake Oswego, OR), and is the smallest Magnetic Resonance (MR) conditional QuadriPolar (QP), Cardiac Resynchronization Therapy Pacemaker (CRT-P) available in the US.
The device features MRI AutoDetect technology, and Closed Loop Stimulation (CLS). The BIOTRONIK Home Monitoring includes Heart Failure Monitor Online functionality that provides a thoracic impedance data and other long-term heart failure statistics. Daily updates enable clinicians to monitor and evaluate patients’ heart failure status throughout the day, and view statistical trends. BIOTRONIK Home is also the only cardiac-device remote monitoring system available with clinically proven improved health outcomes for heart failure patients.
Chicago-based cardiac electrophysiologist, Dr. Roderick Tung, said, “Patient care is a constant journey. It doesn’t end when the patient leaves my office or recovers from a procedure. As healthcare providers, we must think beyond today and help ensure patients are appropriately cared for throughout their lifetime. MR conditional CRT-Ps that can be programmed to automatically switch to MRI mode when they enter the MRI environment are another significant step in delivering the best possible care throughout the patient journey. This technology eliminates an office visit for patients and decreases administrative burden for providers. The impact is significant, especially in institutions that perform cardiac MRI for advanced ventricular care."