New 3T MRI Scanner Cleared by US FDA
By MedImaging International staff writers
Posted on 15 Aug 2017
The US FDA has approved a new wide-bore 3.0T MRI system, developed as part of a four-year collaboration between the US National Football League, mTBI researchers, and a medical imaging device manufacturer.Posted on 15 Aug 2017
The new Magnetic Resonance Imaging (MRI) system offers improved clinical performance, and both neurology and oncology research-focused capabilities to help researchers find new biomarkers for diagnosing mild Traumatic Brain Injury (mTBI).
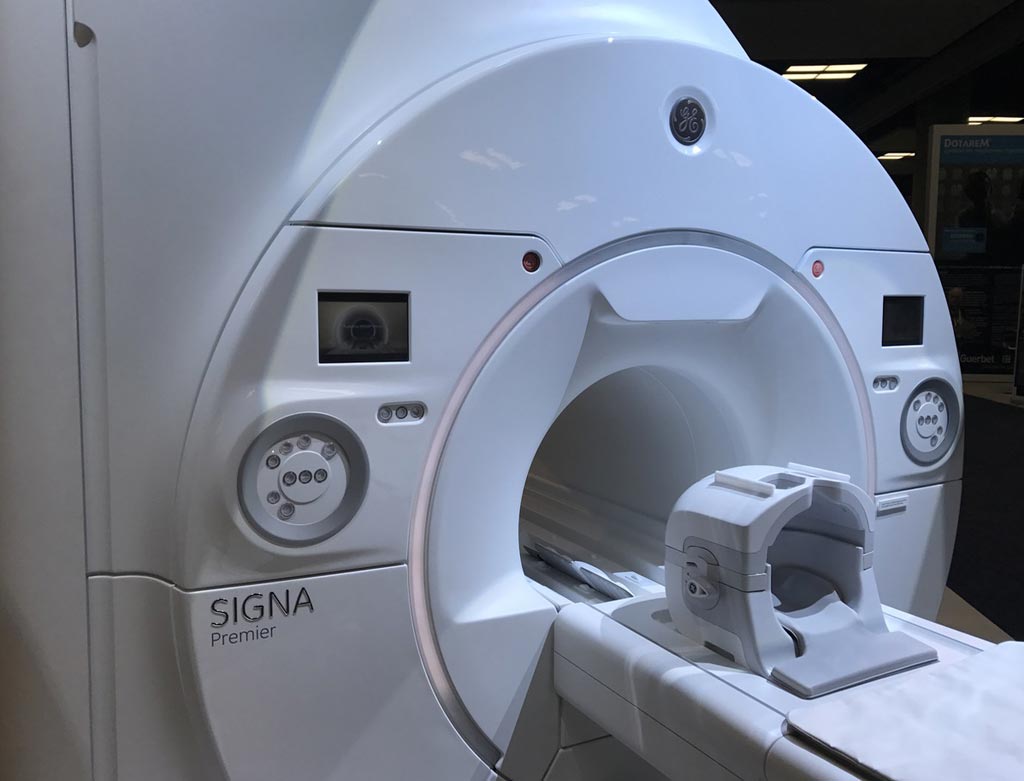
Image: The SIGNA Premier MRI system (Photo courtesy of GE Healthcare).
The SIGNA Premier MRI system is made by GE Healthcare (Chalfont St Giles, Buckinghamshire, UK), and features GE’s newest short-bore, high-homogeneity 3T superconductive magnet, a new digital RF transmit and receive architecture, and GE’s SuperG gradient technology. The SuperG gradient coil provides improved performance and stability. The scanner also features machine-learning software with cloud analytics.
The Radio Frequency (RF) technology of the scanner provides 146 independent receiver channels for faster scanning, improved performance, and improved image quality. The SIGNA Premier scanner also includes a fit-adaptable 48-channel Head Coil with good image quality, and a high Signal-to-Noise Ratio (SNR). The system can perform a routine fast brain exam in less than five minutes using GE’s new HyperSense speed scanning tool that is part of GE’s HyperWorks application suite.
President, and CEO of GE Healthcare MRI, Eric Stahre, said, "We are thrilled to bring SIGNA Premier to clinicians. We believe that its advanced applications and breakthrough innovations will deliver research-focused clinical capabilities and wide-bore patient comfort. This new system will help clinicians push the boundaries of what’s possible with MR."