FDA Clears Specialty Applications for Radiology Platform
By MedImaging International staff writers
Posted on 03 Jul 2017
The US FDA has provided 510(k) clearance for new oncology, neurology and cardiology advanced applications for image comparison and analysis, and evaluation treatments and therapy response assessment.Posted on 03 Jul 2017
The latest release of the clinical informatics platform now includes new advanced clinical applications for multi-modality tumor tracking, optimized lung-nodule assessment, and longitudinal brain imaging.
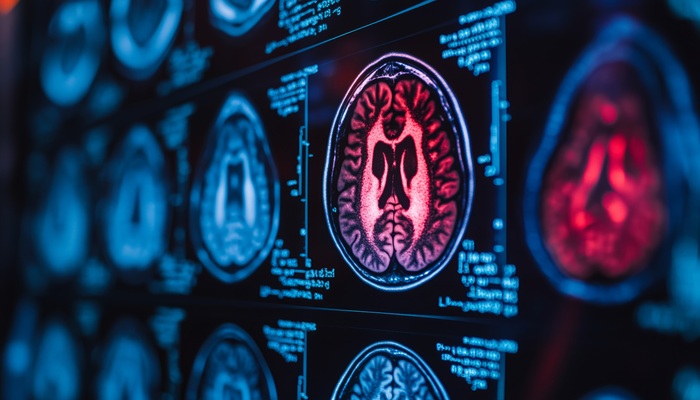
Image: The Longitudinal Brain Imaging (LoBI) is the newest Intellispace 9.0 portal clinical application approved US distribution (Photo courtesy of Philips Healthcare).
The new applications are for the Royal Philips (Amsterdam, the Netherlands) IntelliSpace Portal 9.0 portal, and are now available for marketing in the US. The most recent innovation cleared by the FDA was the Longitudinal Brain Imaging (LoBI) application that can be used to analyze brain images for tracking neurodegenerative disorders including stroke, Multiple Sclerosis (MS), and Alzheimer's disease. New oncology functionality includes qEASL, part of the Multi-Modality Tumor Tracking application, for enhanced tumor volume measurements using Magnetic Resonance Imaging (MRI) and Computed Tomography (CT) scans. The new Lung Nodule Assessment tool provides information about lung nodules from a single CT study, and can also track them across multiple studies.
The IntelliSpace Portal 9.0 portal already offers more than 70 radiology, cardiology, oncology, and neurology applications, and provides a comprehensive overview of a patient’s health. The new applications can enable clinicians to evaluate patients faster, across modalities, and to track therapy response over time.
Leiden University Medical Center professor of neuroradiology, Mark van Buchem, said, "Analytics applications optimized for clinical decision support and longitudinal and quantified patient tracking are becoming increasingly important to radiologists. They can help visualize and quantify very subtle manifestations of disease and differences over time that may not be seen with the naked eye. IntelliSpace Portal 9.0 integrates into our existing workflow and adds greatly to our patient care."










 Guided Devices.jpg)