The World’s First MRI-Safe Quadripolar CRT-D Device Released in Japan
By MedImaging International staff writers
Posted on 13 Jul 2015
A leading manufacturer of cardiovascular medical technology has launched the world’s first Magnetic Resonance Imaging (MRI)-safe quadripolar Cardiac Resynchronization Therapy Defibrillator (CRT-D) in the Japanese market.Posted on 13 Jul 2015
Patients with cardiac devices cannot normally be scanned using existing standard MRI imaging systems, because of the potential damaging effect of strong magnetic fields and radio waves on the devices and their leads.

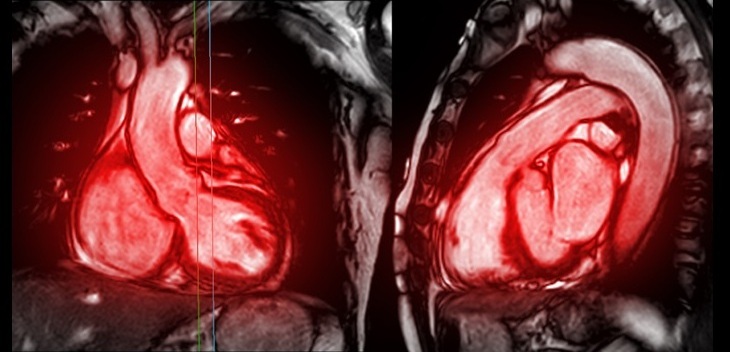
Image: Itrevia 7 HF-T QP Cardiac Resynchronization Therapy Device (Photo courtesy of Biotronik).
The Itrevia 7 HF-T QP device was developed by BIOTRONIK (Berlin, Germany) and features ProMRI technology, for use in MRI scanners. The release of the Itrevia 7 in Japan, the world’s largest MRI market, is part of a wider rollout of BIOTRONIK products in Japan in 2015. BIOTRONIK has the world’s largest portfolio of MR conditional devices, and leads.
Dr. Kenji Ando from Kokura Memorial Hospital, said, “Patients implanted with CIEDs can have a variety of underlying diseases such as sick sinus syndrome, complete AV block, atrial fibrillation with bradycardia, ventricular arrhythmias and/or chronic heart failure. So, it is important that the implanted device is compatible with MRI scans as the risk of stroke is always present. The Itrevia 7 HF-T QP is the first device on the market to combine the benefits of both MRI scans and the quadripolar lead, which makes it a much anticipated device for improving the standard of patient care in Japan.”
Related Links:
BIOTRONIK