Cutting-Edge Imaging Platform Detects Residual Breast Cancer Missed During Lumpectomy Surgery
Posted on 19 Apr 2024
Breast cancer is becoming increasingly common, with statistics indicating that 1 in 8 women will develop the disease in their lifetime. Lumpectomy remains the predominant surgical intervention for treating breast cancer, yet surgeons frequently face challenges in completely removing the tumor in the initial surgery. Current intraoperative tools are insufficient for accurately delineating the tumor boundary, leading to incomplete tumor resections. As a result, up to 36% of patients undergo a second surgery, though in up to 65% of these cases, no residual cancer is found, raising concerns about the need for such additional surgeries due to potential false positive margin assessments or missed cancer in the follow-up procedure. Now, a new technology that is clinically proven to help physicians achieve a more complete cancer resection during lumpectomy could help prevent a second surgery for some patients.
Lumicell’s (Newton, MA, USA) LumiSystem, comprising LUMISIGHT (pegulicianine) optical imaging agent and Lumicell Direct Visualization System (DVS), has an 84% diagnostic accuracy in detecting residual cancer, in real-time, that could be otherwise missed during lumpectomy surgery, while helping some patents avoid second surgeries. The LumiSystem combination is indicated for fluorescence imaging in adults with breast cancer as an adjunct for the intraoperative detection of cancerous tissue within the resection cavity following the removal of the primary specimen during lumpectomy surgery.
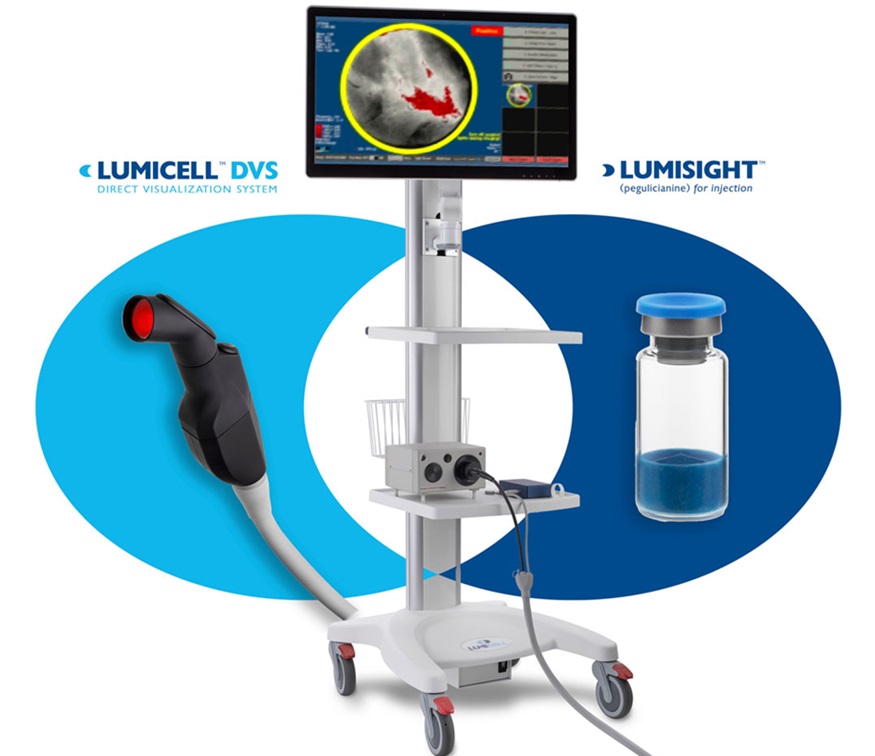
The system’s efficacy and safety have been confirmed through data gathered from over 700 breast cancer patients participating in five clinical studies across leading academic and regional community cancer centers in the U.S. Common side effects associated with LUMISIGHT include hypersensitivity reactions and discoloration of the urine. There are risks of severe hypersensitivity reactions, including anaphylaxis. The U.S. Food & Drug Administration (FDA) has now approved the company’s New Drug Application (NDA) for LUMISIGHT and its Premarket Approval (PMA) application for Lumicell DVC. LUMISIGHT and Lumicell DVS had previously received FDA Fast Track and Breakthrough Device designations, respectively.
“We are immensely proud of the dual approval of LUMISIGHT and Lumicell DVS – we believe this is the first drug-device combination product approved in over a decade to have followed both of the FDA's most stringent NDA and PMA review processes,” said Howard Hechler, President and Chief Operating Officer, Lumicell. “With the FDA’s approval, LumiSystem is now the first and only imaging combination product capable of detecting cancerous tissue where it matters most, inside the breast cavity.”














