Protein Clumping May Contribute to HF Development
By MedImaging International staff writers
Posted on 22 May 2018
A new positron emission tomography (PET) study reveals that cardiac preamyloid oligomers (PAOs) form clumps in developing heart failure (HF).Posted on 22 May 2018
Researchers at the Medical University of Vienna (MedUni; Austria), Johns Hopkins University (JHU; Baltimore, MD, USA), and other institutions conducted a study to see if the mono-phosphorylated protein desmin acts as the seed nucleating PAOs found in human HF. To do so, they used a fluorescent antibody and an amyloid fluorescent stain to visualize and quantify protein clumps in heart tissue biopsies from people with or without HF. They then used a common mouse model of HF to look for desmin clumps. The model involves surgical constriction of the aorta, which raises blood pressure and stress.
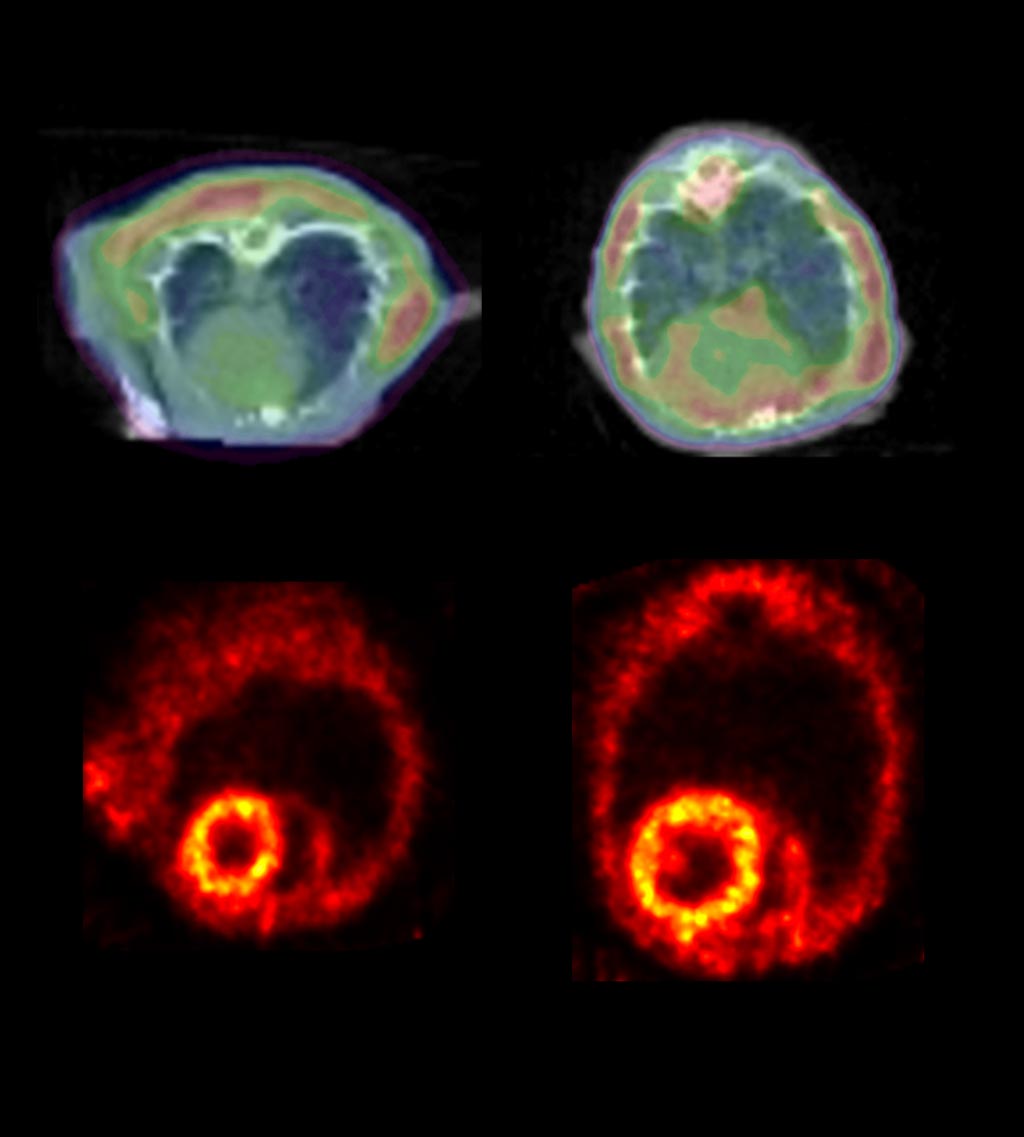
Image: A PET scan detects clumping proteins in HF (R) (Photo courtesy of Johns Hopkins University).
After four weeks, the mice develop symptoms of HF, such as an enlarged heart and lung congestion. The researchers used the radioactive dye Amyvid to image the protein clumps by PET. The results showed that desmin amyloid was more than doubled in the HF mice, when using the same antibody and staining techniques used for the human tissue samples. Conversely, when the researchers used epigallocatechin gallate (EGCG) to break up amyloid, the number of protein clumps halved. The study was published on May 11, 2018, in Circulation Research.
“PET imaging of protein clumps may be eventually used in patients to identify structural changes in the heart as the disease progresses, and this information likely holds prognostic value,” said lead author Peter Rainer, MD, PhD, of the Medical University of Graz. “It could be used as a nice measure of the effect of an intervention to halt or reverse disease progression.”
“From a molecular standpoint there’s not a unified, clear mechanism for why the heart goes into failure. But by figuring out this mechanism, we may be able to devise better treatments and diagnostic tools,” said senior author Giulio Agnetti, PhD, of JHU. “I think the next step is to follow up with the proteins that are dynamically modified in response to environment, which places a larger emphasis on lifestyle intervention to help prevent diseases.”
Related Links:
Medical University of Vienna
Johns Hopkins University














