FDA Approves Ultrasound Aimed at Liver Tissue Assessment
By MedImaging International staff writers
Posted on 21 Mar 2017
The US Food and Drug Administration (FDA) 510(k) has approved the use of new Ultrasound Shear Wave Elastography (SWE) functionality, with an existing family of ultrasound systems, for liver tissue-stiffness assessment.Posted on 21 Mar 2017
The new functionality will enable simultaneous imaging of tissue together with stiffness assessment, and will help clinicians diagnosis various liver conditions such as hepatitis B and C, cirrhosis of the liver, and liver cancer.
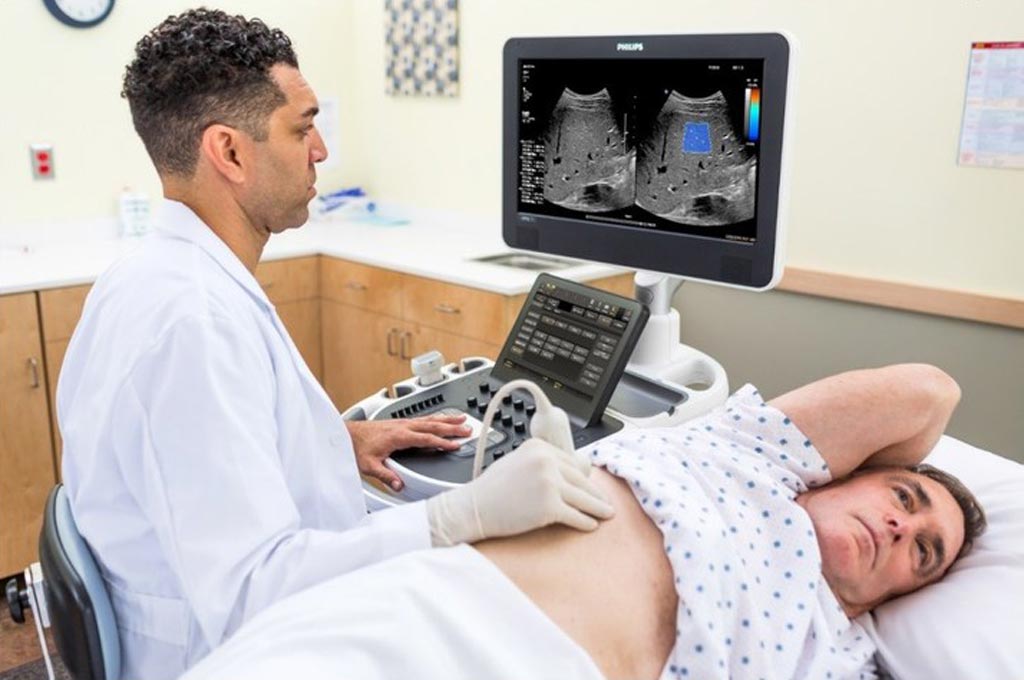
Image: Liver disease assessment using the ultrasound ElastQ Imaging SWE functionality is faster and than a tissue biopsy, and noninvasive (Photo courtesy of Philips Healthcare).
The US FDA approved the new SWE functionality the ElastQ ultrasound system manufactured by Royal Philips. ElastQ imaging provides a non-invasive, reproducible, and comprehensive solution for the assessment and diagnosis of liver conditions. Ultrasound SWE technology could in the future become routine for assessing the status of liver disease in patients, and could help reduce or even avoid the need for painful invasive liver biopsies.
ElastQ Imaging shear wave elastography provides a large Region of Interest (ROI), color-coded quantitative tissue-stiffness assessment, real-time feedback, intelligent analysis, and quantitative measurements using multiple sample points.
Ultrasound Business Leader at Philips, Vitor Rocha, said, "Philips aims to provide the tools necessary for assessing and managing chronic conditions that so many people face, and liver disease is no exception. We know that liver disease is a growing health concern around the globe, and we are committed to pioneering innovations like ElastQ Imaging to create our ultimate ultrasound liver solution that offers exceptional clinical performance, further improving patient care."