Pushing the Boundaries with CEUS and US Elastography
By MedImaging International staff writers
Posted on 14 Feb 2017
Ultrasound elastography and contrast enhanced ultrasound (CEUS) are among the latest advances in ultrasound (US) technology that offer improved spatial and temporal resolution in the detection and characterization of abnormal tissues. SonoScape is leading the field in developing this technology that is improving outcomes for patients.Posted on 14 Feb 2017
Leader in the field, Andrej Lyshchik, M.D., Ph.D., Assistant Professor at the Department of Radiology at Thomas Jefferson University Hospital in Philadelphia, US, is enthusiastic for SonoScape’s US elastography. He welcomed the nature of US elastography as a non-invasive technique that allows detection and characterization of tissues with abnormal biomechanical properties.
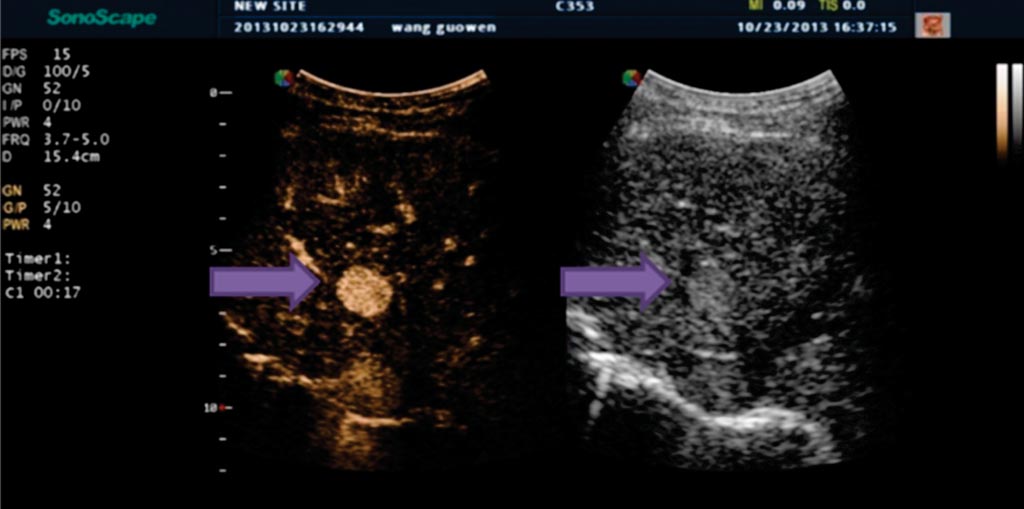
Image: Indeterminate liver lesion in patient with cirrhosis (arrow) demonstrating avid arterial phase hyper-enhancement, suspicious for HCC (Photo courtesy of Andrej Lyshchik, M.D., Ph.D., Thomas Jefferson University Hospital).
US Elastography
Currently, there are three main types of US elastography: transient elastography, compression elastography and Acoustic Radiation Force Impulse (ARFI) imaging. Transient elastography uses an external device that generates mechanical displacement of tissues and a built-in US transducer to register shear wave that propagates within the examined tissues. ARFI imaging uses acoustic radiation forces to generate tissue displacement and map its elastic properties based on the speed of sheer wave propagation.
To date, liver, kidney, breast and prostate are among the organs that show most benefit from these technologies. In particular, Dr Lyshchik explained that one of the most commonly used clinical applications of ARFI and transient elastography is for the evaluation of patients with chronic liver disease.
CEUS in the Evaluation Of Tumor Blood Flow
Likewise, CEUS also promises improvements in patient management with its high resolution, real-time visualization of new blood vessels within tumors that increases diagnostic accuracy. CEUS can be used on SonoScape’s scanners.
CEUS uses US contrast agents that are composed of a gas microbubbles, encapsulated by an outer protein or lipid shell. These microbubbles are of a diameter (1-8 µm) that enables passage through the pulmonary capillaries, but restricts the microbubbles to the vasculature, making them excellent intravascular blood pool agents. Unlike contrast agents for MRI and CT, US contrast agents are not nephrotoxic and have no renal contraindications, making them an exceptionally safe to use.
Detecting such changes in tumor vascularity (including blood flow kinetics and microvascular density) is a recognized indicator of treatment response via visualization of the perfusion of US contrast agents. Parameters such as the time required from injection to contrast arrival, rate of US contrast agent inflow, rate of US contrast agent washout, and cumulative US contrast agents signal over time (an indicator of net blood flow) have all been shown to be potentially useful indicators of treatment response. CEUS also provides the opportunity to create 3D parametric maps of tumor perfusion to illustrate the differences in intra-tumoral blood flow kinetics.
Dr Lyshchik explained that within oncology, CEUS can detect changes in tumor vascularity that provide an indicator of treatment response to certain therapies. He remarked that due to the real time nature of ultrasound and the blood pooling properties of US contrast agents, visualization of UCA perfusion provides an indicator of the blood flow kinetics and microvascular density of the tumor.
CEUS of Liver Nodules and Hepatocellular Carcinoma
Of note, CEUS is also a potentially safer, less expensive and more readily available technique for characterizing focal liver nodules in patients at risk for hepatocellular carcinoma, compared to the current clinical standard. This significant cancer, rated as the fifth most common cancer worldwide with an annual incidence of over 550,000, predominantly affects patients with cirrhosis and chronic hepatitis.
But imaging to diagnose hepatocellular carcinoma can be challenging, especially in patients with advanced cirrhosis, in which structural and physiological alterations of the liver can impair detection of the cancer. However, studies of CEUS, which is available on SonoScape’s scanners in this capacity, have demonstrated safety, high specificity and positive predictive value for diagnosis of hepatocellular carcinoma compared to hepatobiliary agent gadoxetate-enhanced MRI.
In an effort to facilitate the clinical use of CEUS, the American College of Radiology recently introduced the CEUS Liver Imaging Reporting and Data System (CEUS LI-RADS), which provides standardisation of CEUS examination and reporting, and allows liver nodule classification based on their likelihood to be hepatocellular carcinoma.
Finally, worth a mention is CEUS-guided biopsy, which is another application of SonoScape’s technology that targets and biopsies lesions, normally invisible or hard to detect, for example, the small nodules of hepatocellular carcinoma on cirrhosis or adenocarcinoma’s areas in the prostate. It can also target viable areas of large, necrotic tumors.






 Guided Devices.jpg)